Technical Notes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hyperparathyroidism
HYPERPARATHYROIDISM Edited by Gonzalo Díaz-Soto and Manuel Puig-Domingo Hyperparathyroidism Edited by Gonzalo Díaz-Soto and Manuel Puig-Domingo Published by InTech Janeza Trdine 9, 51000 Rijeka, Croatia Copyright © 2012 InTech All chapters are Open Access distributed under the Creative Commons Attribution 3.0 license, which allows users to download, copy and build upon published articles even for commercial purposes, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. After this work has been published by InTech, authors have the right to republish it, in whole or part, in any publication of which they are the author, and to make other personal use of the work. Any republication, referencing or personal use of the work must explicitly identify the original source. As for readers, this license allows users to download, copy and build upon published chapters even for commercial purposes, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. Notice Statements and opinions expressed in the chapters are these of the individual contributors and not necessarily those of the editors or publisher. No responsibility is accepted for the accuracy of information contained in the published chapters. The publisher assumes no responsibility for any damage or injury to persons or property arising out of the use of any materials, instructions, methods or ideas contained in the book. Publishing Process Manager Romana Vukelic Technical Editor Teodora Smiljanic Cover Designer InTech Design Team First published April, 2012 Printed in Croatia A free online edition of this book is available at www.intechopen.com Additional hard copies can be obtained from [email protected] Hyperparathyroidism, Edited by Gonzalo Díaz-Soto and Manuel Puig-Domingo p. -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -

1 Annex 2. AHRQ ICD-9 Procedure Codes 0044 PROC-VESSEL
Annex 2. AHRQ ICD-9 Procedure Codes 0044 PROC-VESSEL BIFURCATION OCT06- 0201 LINEAR CRANIECTOMY 0050 IMPL CRT PACEMAKER SYS 0202 ELEVATE SKULL FX FRAGMNT 0051 IMPL CRT DEFIBRILLAT SYS 0203 SKULL FLAP FORMATION 0052 IMP/REP LEAD LF VEN SYS 0204 BONE GRAFT TO SKULL 0053 IMP/REP CRT PACEMAKR GEN 0205 SKULL PLATE INSERTION 0054 IMP/REP CRT DEFIB GENAT 0206 CRANIAL OSTEOPLASTY NEC 0056 INS/REP IMPL SENSOR LEAD OCT06- 0207 SKULL PLATE REMOVAL 0057 IMP/REP SUBCUE CARD DEV OCT06- 0211 SIMPLE SUTURE OF DURA 0061 PERC ANGIO PRECEREB VES (OCT 04) 0212 BRAIN MENINGE REPAIR NEC 0062 PERC ANGIO INTRACRAN VES (OCT 04) 0213 MENINGE VESSEL LIGATION 0066 PTCA OR CORONARY ATHER OCT05- 0214 CHOROID PLEXECTOMY 0070 REV HIP REPL-ACETAB/FEM OCT05- 022 VENTRICULOSTOMY 0071 REV HIP REPL-ACETAB COMP OCT05- 0231 VENTRICL SHUNT-HEAD/NECK 0072 REV HIP REPL-FEM COMP OCT05- 0232 VENTRI SHUNT-CIRCULA SYS 0073 REV HIP REPL-LINER/HEAD OCT05- 0233 VENTRICL SHUNT-THORAX 0074 HIP REPL SURF-METAL/POLY OCT05- 0234 VENTRICL SHUNT-ABDOMEN 0075 HIP REP SURF-METAL/METAL OCT05- 0235 VENTRI SHUNT-UNINARY SYS 0076 HIP REP SURF-CERMC/CERMC OCT05- 0239 OTHER VENTRICULAR SHUNT 0077 HIP REPL SURF-CERMC/POLY OCT06- 0242 REPLACE VENTRICLE SHUNT 0080 REV KNEE REPLACEMT-TOTAL OCT05- 0243 REMOVE VENTRICLE SHUNT 0081 REV KNEE REPL-TIBIA COMP OCT05- 0291 LYSIS CORTICAL ADHESION 0082 REV KNEE REPL-FEMUR COMP OCT05- 0292 BRAIN REPAIR 0083 REV KNEE REPLACE-PATELLA OCT05- 0293 IMPLANT BRAIN STIMULATOR 0084 REV KNEE REPL-TIBIA LIN OCT05- 0294 INSERT/REPLAC SKULL TONG 0085 RESRF HIPTOTAL-ACET/FEM -

California Breast Cancer Research Program Special Research Initiatives
UC Office of the President UCOP Previously Published Works Title Identifying gaps in breast cancer research: Addressing disparities and the roles of the physical and social environment Permalink https://escholarship.org/uc/item/02p5s6xr Publication Date 2007 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Identifying Gaps in Breast Cancer Research California Breast Cancer Research Program Special Research Initiatives Identifying gaps in breast cancer research: Addressing disparities and the roles of the physical and social environment Editors Julia G. Brody, PhD Executive Director Silent Spring Institute Marion (Mhel) H.E. Kavanaugh-Lynch, MD, MPH Director California Breast Cancer Research Program Olufunmilayo I (Funmi) Olopade, MD Walter L. Palmer Distinguished Service Professor of Medicine University of Chicago Medical Center Susan Matsuko Shinagawa Breast Cancer and Chronic Pain Survivor/Advocate, Intercultural Cancer Council; Asian and Pacific Islander National Cancer Survivors Network Sandra Steingraber, PhD Author and Distinguished Visiting Scholar Ithaca College David R. Williams, PhD Department of Society, Human Development and Health Harvard School of Public Health Front Matter DRAFT 8/11/07 Page 1 California Breast Cancer Research Program Table of Contents Preface Introduction Section I: Exposures from the Physical Environment and Breast Cancer Overarching Issues A. Secondhand Smoke B. Environmental Chemicals/Pollutants 1. Air Pollutants, Fuels and Additives 2. Persistent Organic Pollutants 3. Polybrominated Flame Retardants 4. Pesticides 5. Solvents and industrial chemicals 6. Water Contaminants 7. Hormones in Food 8. Metals 9. Exposures from Polyvinyl Chloride 10.Bisphenol A C. Compounds in Personal Care Products D. Pharmaceuticals E. Infectious agents F. Ionizing Radiation G. -

The Relationship Between Bone Metabolism, Melatonin and Other Hormones in Sham-Operated and Pinealectomized Rats
ENDOCRINE REGULATIONS, VOL. 37, 211–224, 2003 211 THE RELATIONSHIP BETWEEN BONE METABOLISM, MELATONIN AND OTHER HORMONES IN SHAM-OPERATED AND PINEALECTOMIZED RATS Z. OSTROWSKA, B. KOS-KUDLA1, M. NOWAK1, E. SWIETOCHOWSKA, B. MAREK1, J. GORSKI, D. KAJDANIUK1, K. WOLKOWSKA Department of Clinical Biochemistry, 1Department of Pathophysiology and Endocrinology, The Medical University of Silesia, Zabrze, Poland E-mail: [email protected] Objective. The influence of pinealectomy and long-term melatonin (MEL) administration on circadian oscillations of selected biochemical markers of bone metabolism [serum alkaline phos- phatase (ALP) activity, carboxyterminal propeptide type I procollagen (PICP) and carboxyterminal telopeptide type I collagen (ICTP) concentrations as well as urinary excretion of hydroxyproline (HYP) and Ca] and possible involvement of circadian secretion of IGF-I, parathyroid, thyroid, adrenal cortex and gonads function in this mechanism was evaluated. Methods. Studies were performed in 192 adult male Wistar rats weighing 145±9 g which were subjected to pinealectomy or sham operation. In half of the animals from each group MEL (Sigma, USA) in a dose of 50 mg/100 g b.w. was administered intraperitonealy (daily between 17.00 and 18.00 h for a 4-week period). Material for studies (blood and urine) was collected every 3 hours during a day. Hormones, PICP and ICTP concentrations were determined with the use of RIA meth- ods, whereas ALP, HYP and Ca values - spectrophotometrically. Results. The study has shown that pinealectomy had an inducing, while exogenous MEL a suppressing effect upon the level of investigated biochemical markers of bone metabolism. Fur- thermore, substantial changes in the values of amplitude and phase of their circadian oscillations were shown. -
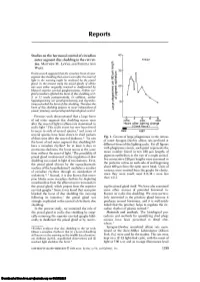
Studies on the Hormonal Control of Circadian Outer Segment Disc
Reports Studies on the hormonal control of circadian 40 outer segment disc shedding in the rat ret- • Intact ina. MATTHEW M. LA VAIL AND PATRICIA ANN c WARD. 30 Previous work suggested that the circadian burst of outer segment disc shedding that occurs soon after the onset of 8| light in the morning might be mediated by the pineal gland. In the present study the pineal glands of albino rats were either surgically removed or deafferented by 1= 20 bilateral superior cervical ganglionectomy. Neither sur- gical procedure affected the burst of disc shedding at 2, 3, or 11 weeks postoperatively. In addition, neither hypophysectomy nor parathyroidectomy and thyroidec- 10 tomy perturbed, the burst of disc shedding. Therefore the burst of disc shedding appears to occur independent of pineal, pituitary, and parathyroid-thyroid gland control. Previous work demonstrated that a large burst 0 2 4 6 8 of rod outer segment disc shedding occurs soon (0700) (1100) (1500) after the onset of light in albino rats maintained in Hours after lighting change (Clock hours) cyclic light.' This cyclic event has now been found M 1 to occur in rods of several species,2 and cones of Dark Light several species have been shown to shed packets Fig. 1. Counts of large phagosomes in the retinas of discs soon after the onset of darkness.3'4 In rats of intact Sprague-Dawley albino rats perfused at the burst of rod outer segment disc shedding fol- different times of the lighting cycle. For all figures lows a circadian rhythm* for at least 3 days in with phagosome counts, each point represents the continuous darkness; the burst occurs at the same mean number found in ten 180 /am lengths of time without the onset of light.' The possibility of pigment epithelium in the eye of a single animal, pineal gland involvement in the regulation of disc five consecutive 180 /am lengths were examined in shedding was raised in light of two features. -

Psi Technical Specs V30a.Pdf
AHRQ Quality Indicators Patient Safety Indicators: Technical Specifications Department of Health and Human Services Agency for Healthcare Research and Quality http://www.qualityindicators.ahrq.gov March 2003 Version 3.0a (May 1, 2006) AHRQ Quality Indicators Web Site: http://www.qualityindicators.ahrq.gov Table of Contents Complications of Anesthesia (PSI 1) ............................................................................................................ 1 Death in Low-Mortality DRGs (PSI 2) ........................................................................................................... 3 Decubitus Ulcer (PSI 3) ................................................................................................................................. 5 Failure to Rescue (PSI 4) .............................................................................................................................. 7 Foreign Body Left during Procedure, Secondary Diagnosis Field (PSI 5 and 21)...................................... 14 Iatrogenic Pneumothorax, Secondary Diagnosis Field (PSI 6 and 22)....................................................... 15 Selected Infections Due to Medical Care, Secondary Diagnosis Field (PSI 7 and 23) .............................. 21 Postoperative Hip Fracture (PSI 8) ............................................................................................................. 21 Postoperative Hemorrhage or Hematoma (PSI 9 and 27) ......................................................................... -

Surgeries Performed in Rodents in North America
RESEARCH MODELS Surgical Services - North America At Charles River we offer a variety of surgical services on five species (rat, mouse, guinea pig, hamster, and gerbil) at four different AAALAC-accredited production facilities in the United States and multiple international sites. Our surgical services are conducted by highly skilled surgeons with vast experience in veterinary medicine and animal science and range from simple procedures (e.g., soft tissue/organ extractions) to highly complex catheterizations and cannulations. We now offer more than 80 surgical procedures, all of which meet exacting scientific and regulatory standards. We continue to expand our capabilities, reflecting our ongoing commitment to providing the surgically altered animal models that meet your research needs. State-of-the-Art Facilities North American Surgical Locations Charles River currently offers full surgical capabilities in several • Hollister, California AAALAC-accredited facilities in North America. The surgical suites • Kingston, New York at each site are composed of HEPA-filtered, positive-pressure • Raleigh, North Carolina barrier rooms with a series of entry locks. Each suite features • Wilmington, Massachusetts individual functional areas used for surgical manipulations, animal husbandry support, preoperative holding, postoperative recovery, Capabilities supply preparation, and clerical activities. • Vascular catheterizations • Non-vascular catheterizations Biosecurity • Cardiovascular procedures To ensure strict adherence to biosecurity policies, animals are • Neurological procedures not transferred between barrier production facilities, nor are non- Charles River animals used. Additional biosecurity measures • Soft tissue procedures include barrier-type surgical suites, the use of dedicated laminar • Device implants flow workstations by each surgeon, and housing of animals in filter- • Customized procedures top caging systems during the animals’ stay in surgery. -

The Effect of Bilateral Nephrectomy and Midcollicular De- Cerebration
Journal of Clinical Investigation Vol. 43, No. 8, 1964 The Effect of Bilateral Nephrectomy and Midcollicular De- cerebration with Pinealectomy and Hypophysectomy on the Corticosteroid Secretion of Sodium- deficient Sheep * J. R. BLAIR-WEST, J. P. COGHLAN, D. A. DENTON, J. A. MUNRO, MARELYN WINTOUR, AND R. D. WRIGHT (From the Howard Florey Laboratories of Experimental Physiology, University of Melbourne, Parkville, N.2., Australia) The adrenal gland, whether in its normal situa- periments studying the onset of sodium deficiency tion or transplanted to another site, responds to (2) and inferior vena cava constriction (15), it sodium depletion (1, 2), constriction of the in- has been shown that aldosterone hypersecretion ferior vena cava (3), or hemorrhage (3, 4) by in- may occur without or preceding any change of ar- creased secretion of aldosterone. Crosscircula- terial plasma electrolyte concentration. tion studies of Denton, Goding, and Wright (1) Davis and associates (16) and Ganong and and Yankopoulos, Davis, Kliman, and Peterson Mulrow (17) have reported that dogs with hypo- (5) indicate that the stimulus to the adrenal is physectomy and nephrectomy showed no aldos- humoral. Three local humoral circumstances, terone response to hemorrhage, and Davis, Ayers, ACTH infusion (2, 6-8), angiotensin II infusion and Carpenter (18) report nephrectomy caused (2, 6, 9-12), and low sodium and high potassium an 80%o reduction of aldosterone and corticos- concentration of plasma (1, 2, 6, 13), are known terone response to caval constriction and sodium to cause an increase in aldosterone secretion, and depletion in hypophysectomized dogs. the levels required for the reaction are not beyond There are a number of conflicting reports con- those that are known or might be presumed to oc- cerning the influence of ablations of the pineal and cur naturally. -

Fill in the Blank Key
MEDICAL TERMINOLOGY THE ENDOCRINE SYSTEM FILL IN THE BLANK KEY ANATOMICAL TERMS 1. Antidiuretic hormone (ADH) Hormone influencing absorption of water by kidneys 2. Adrenal cortex Outer part of kidney gland 3. Oxytocin Hormone that stimulates parturition 4. Prolactin Hormone that stimulates milk production 5. Adrenal medulla Inner part of kidneys gland 6. Insulin Hormone produced by islets of Langerhans 7. Estrogen Hormone that puts women “in heat” 8. Melanocyte-stimulating hormone Hormone that affects skin pigmentation 9. Epinephrine Hormone produced by kidney marrow 10. Testosterone Hormone produced by male gonads 1. Cell clusters in pancreas producing insulin Islets of Langerhans 2. Hormone assisting body to deal with stress Epinephrine 3. Hormone stimulating skin pigment Melanocyte stimulating hormone 4. Hormone stimulating labor pains Oxytocin 5. Four small bodies directly behind the thyroid Parathyroids 6. Outer part of gland above each kidney Adrenal corte 7. Inner marrowy part of gland above each Adrenal medulla kidney 8. Hypophysis Pituitary 9. Hormone that converts glycogen to glucose Glucagon 10. Male gonads Testes SYMPTOMATIC AND DIAGNOSTIC TERMS 1. Glycosuria Sugar in urine 2. Hypercalcemia Excessive calcium in blood 3. Hypokalemia Deficient potassium in blood 4. Polydipsia Excessive thirst 5. Adrenal virilism Excessive production of androgen in women 6. Diabetes mellitus Hyperglycemia and glucosuria 7. Adrenomegaly Enlargement of kidney’s gland 8. Adenopathy Any disease of a gland 9. Thyrotoxicosis A toxic condition of thyroid because of its hyperactivity 10. Hirsutism Excessive growth of hair, especially in women 11. Exophthalmia Eyeball protusion 1. Deficiency of sugar in the blood Hypoglycemia 2. Excess of calcium in the blood Hypercalcemia 3. -

Operating Room Procedure Codes
Operating Room Procedure Codes The ICD-9-CM codes added in PSI version 2.1, revision 3 (January 17, 2005) are identified by the date of introduction, e.g., OCT 04, after the code label. 0050 IMPL CRT PACEMAKER SYS 0302 REOPEN LAMINECTOMY SITE 0051 IMPL CRT DEFIBRILLAT SYS 0309 SPINAL CANAL EXPLOR NEC 0052 IMP/REP LEAD LF VEN SYS 031 INTRASPIN NERVE ROOT DIV 0053 IMP/REP CRT PACEMAKR GEN 0321 PERCUTANEOUS CHORDOTOMY 0054 IMP/REP CRT DEFIB GENAT 0329 OTHER CHORDOTOMY 0061 PERC ANGIO PRECEREB VES (OCT 04) 0332 SPINAL CORD/MENINGES BX 0062 PERC ANGIO INTRACRAN VES (OCT 04) 0339 OTHER SPINAL DX PROC 0112 OPEN CEREB MENINGES BX 034 EXCIS SPINAL CORD LESION 0114 OPEN BRAIN BIOPSY 0351 SPINE MENINGOCELE REPAIR 0115 SKULL BIOPSY 0352 MYELOMENINGOCEL REPAIR 0118 OTHER BRAIN DX PROCEDURE 0353 VERTEBRAL FX REPAIR 0119 OTHER SKULL DX PROCEDURE 0359 SPINAL STRUCT REPAIR NEC 0121 CRANIAL SINUS I & D 036 SPINAL CORD ADHESIOLYSIS 0122 REMOV INTRACRAN STIMULAT 0371 SUBARACH-PERITON SHUNT 0123 REOPEN CRANIOTOMY SITE 0372 SUBARACH-URETERAL SHUNT 0124 OTHER CRANIOTOMY 0379 OTH SPINAL THECAL SHUNT 0125 OTHER CRANIECTOMY 0393 INSERT SPINAL STIMULATOR 0131 INCISE CEREBRAL MENINGES 0394 REMOVE SPINAL STIMULATOR 0132 LOBOTOMY & TRACTOTOMY 0397 REVISE SPINE THECA SHUNT 0139 OTHER BRAIN INCISION 0398 REMOVE SPINE THECA SHUNT 0141 THALAMUS OPERATIONS 0399 SPINE CANAL STRUC OP NEC 0142 GLOBUS PALLIDUS OPS 0401 EXCISION ACOUSTC NEUROMA 0151 EX CEREB MENINGEAL LES 0402 TRIGEMINAL NERV DIVISION 0152 HEMISPHERECTOMY 0403 PERIPH NERVE DIV NEC 0153 BRAIN LOBECTOMY 0404 PERIPH -

Medicare Data Utilities Appendices
Medicare Data Utilities Appendices B Beneficiary Primary Payer Table C BETOS Table D Carrier Claim Payment Denial Table E Carrier Line Provider Type Table G CMS Provider Specialty Table H CMS Type of Service Table I Line Place of Service Table J NCH Edit Table K NCH Patch Table L State Table M Identification of variables used in summary level aggregation N Agency for Healthcare Research and Quality (AHRQ)Diagnostic Codes O Agency for Healthcare Research and Quality (AHRQ)Procedure Codes P International Classification of Diseases (ICD-9) Diagnostic Codes (v18) Q International Classification of Diseases (ICD-9) Surgery Codes (v18) R Diagnostic Related Group (DRG) Number Table (v18) S Alpha-Numeric HCPCS Codes Appendix B Beneficiary Primary Payer Table The following claim-level variables reference this Appendix: bprpycd Line beneficiary primary payer code A = Working aged bene/spouse with employer group health plan (eghp) B = End stage renal disease (ESRD) beneficiary in the 18 month coordination period with an employer group health plan C = Conditional payment by Medicare; future reimbursement expected D = Automobile no-fault (eff. 4/97; Prior to 3/94, also included any Liability insurance) E = Workers' compensation F = Public Health Service or other federal agency (other than Dept. of Veterans Affairs) G = Working disabled bene (under age 65 with LGHP) H = Black Lung I = Dept. of Veterans Affairs J = Any liability insurance (eff. 3/94 - 3/97) L = Any liability insurance (eff. 4/97) (eff. 12/90 for carrier claims and 10/93 for FI claims; obsoleted for all claim types 7/1/96) M = Override code: EGHP services involved (eff.