Chapter 2: NMR and Energy Levels
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unit 1 Old Quantum Theory
UNIT 1 OLD QUANTUM THEORY Structure Introduction Objectives li;,:overy of Sub-atomic Particles Earlier Atom Models Light as clectromagnetic Wave Failures of Classical Physics Black Body Radiation '1 Heat Capacity Variation Photoelectric Effect Atomic Spectra Planck's Quantum Theory, Black Body ~diation. and Heat Capacity Variation Einstein's Theory of Photoelectric Effect Bohr Atom Model Calculation of Radius of Orbits Energy of an Electron in an Orbit Atomic Spectra and Bohr's Theory Critical Analysis of Bohr's Theory Refinements in the Atomic Spectra The61-y Summary Terminal Questions Answers 1.1 INTRODUCTION The ideas of classical mechanics developed by Galileo, Kepler and Newton, when applied to atomic and molecular systems were found to be inadequate. Need was felt for a theory to describe, correlate and predict the behaviour of the sub-atomic particles. The quantum theory, proposed by Max Planck and applied by Einstein and Bohr to explain different aspects of behaviour of matter, is an important milestone in the formulation of the modern concept of atom. In this unit, we will study how black body radiation, heat capacity variation, photoelectric effect and atomic spectra of hydrogen can be explained on the basis of theories proposed by Max Planck, Einstein and Bohr. They based their theories on the postulate that all interactions between matter and radiation occur in terms of definite packets of energy, known as quanta. Their ideas, when extended further, led to the evolution of wave mechanics, which shows the dual nature of matter -

Quantum Theory of the Hydrogen Atom
Quantum Theory of the Hydrogen Atom Chemistry 35 Fall 2000 Balmer and the Hydrogen Spectrum n 1885: Johann Balmer, a Swiss schoolteacher, empirically deduced a formula which predicted the wavelengths of emission for Hydrogen: l (in Å) = 3645.6 x n2 for n = 3, 4, 5, 6 n2 -4 •Predicts the wavelengths of the 4 visible emission lines from Hydrogen (which are called the Balmer Series) •Implies that there is some underlying order in the atom that results in this deceptively simple equation. 2 1 The Bohr Atom n 1913: Niels Bohr uses quantum theory to explain the origin of the line spectrum of hydrogen 1. The electron in a hydrogen atom can exist only in discrete orbits 2. The orbits are circular paths about the nucleus at varying radii 3. Each orbit corresponds to a particular energy 4. Orbit energies increase with increasing radii 5. The lowest energy orbit is called the ground state 6. After absorbing energy, the e- jumps to a higher energy orbit (an excited state) 7. When the e- drops down to a lower energy orbit, the energy lost can be given off as a quantum of light 8. The energy of the photon emitted is equal to the difference in energies of the two orbits involved 3 Mohr Bohr n Mathematically, Bohr equated the two forces acting on the orbiting electron: coulombic attraction = centrifugal accelleration 2 2 2 -(Z/4peo)(e /r ) = m(v /r) n Rearranging and making the wild assumption: mvr = n(h/2p) n e- angular momentum can only have certain quantified values in whole multiples of h/2p 4 2 Hydrogen Energy Levels n Based on this model, Bohr arrived at a simple equation to calculate the electron energy levels in hydrogen: 2 En = -RH(1/n ) for n = 1, 2, 3, 4, . -

Quantum Field Theory*
Quantum Field Theory y Frank Wilczek Institute for Advanced Study, School of Natural Science, Olden Lane, Princeton, NJ 08540 I discuss the general principles underlying quantum eld theory, and attempt to identify its most profound consequences. The deep est of these consequences result from the in nite number of degrees of freedom invoked to implement lo cality.Imention a few of its most striking successes, b oth achieved and prosp ective. Possible limitation s of quantum eld theory are viewed in the light of its history. I. SURVEY Quantum eld theory is the framework in which the regnant theories of the electroweak and strong interactions, which together form the Standard Mo del, are formulated. Quantum electro dynamics (QED), b esides providing a com- plete foundation for atomic physics and chemistry, has supp orted calculations of physical quantities with unparalleled precision. The exp erimentally measured value of the magnetic dip ole moment of the muon, 11 (g 2) = 233 184 600 (1680) 10 ; (1) exp: for example, should b e compared with the theoretical prediction 11 (g 2) = 233 183 478 (308) 10 : (2) theor: In quantum chromo dynamics (QCD) we cannot, for the forseeable future, aspire to to comparable accuracy.Yet QCD provides di erent, and at least equally impressive, evidence for the validity of the basic principles of quantum eld theory. Indeed, b ecause in QCD the interactions are stronger, QCD manifests a wider variety of phenomena characteristic of quantum eld theory. These include esp ecially running of the e ective coupling with distance or energy scale and the phenomenon of con nement. -

Vibrational Quantum Number
Fundamentals in Biophotonics Quantum nature of atoms, molecules – matter Aleksandra Radenovic [email protected] EPFL – Ecole Polytechnique Federale de Lausanne Bioengineering Institute IBI 26. 03. 2018. Quantum numbers •The four quantum numbers-are discrete sets of integers or half- integers. –n: Principal quantum number-The first describes the electron shell, or energy level, of an atom –ℓ : Orbital angular momentum quantum number-as the angular quantum number or orbital quantum number) describes the subshell, and gives the magnitude of the orbital angular momentum through the relation Ll2 ( 1) –mℓ:Magnetic (azimuthal) quantum number (refers, to the direction of the angular momentum vector. The magnetic quantum number m does not affect the electron's energy, but it does affect the probability cloud)- magnetic quantum number determines the energy shift of an atomic orbital due to an external magnetic field-Zeeman effect -s spin- intrinsic angular momentum Spin "up" and "down" allows two electrons for each set of spatial quantum numbers. The restrictions for the quantum numbers: – n = 1, 2, 3, 4, . – ℓ = 0, 1, 2, 3, . , n − 1 – mℓ = − ℓ, − ℓ + 1, . , 0, 1, . , ℓ − 1, ℓ – –Equivalently: n > 0 The energy levels are: ℓ < n |m | ≤ ℓ ℓ E E 0 n n2 Stern-Gerlach experiment If the particles were classical spinning objects, one would expect the distribution of their spin angular momentum vectors to be random and continuous. Each particle would be deflected by a different amount, producing some density distribution on the detector screen. Instead, the particles passing through the Stern–Gerlach apparatus are deflected either up or down by a specific amount. -

Coupling Constant Unification in Extensions of Standard Model
Coupling Constant Unification in Extensions of Standard Model Ling-Fong Li, and Feng Wu Department of Physics, Carnegie Mellon University, Pittsburgh, PA 15213 May 28, 2018 Abstract Unification of electromagnetic, weak, and strong coupling con- stants is studied in the extension of standard model with additional fermions and scalars. It is remarkable that this unification in the su- persymmetric extension of standard model yields a value of Weinberg angle which agrees very well with experiments. We discuss the other possibilities which can also give same result. One of the attractive features of the Grand Unified Theory is the con- arXiv:hep-ph/0304238v2 3 Jun 2003 vergence of the electromagnetic, weak and strong coupling constants at high energies and the prediction of the Weinberg angle[1],[3]. This lends a strong support to the supersymmetric extension of the Standard Model. This is because the Standard Model without the supersymmetry, the extrapolation of 3 coupling constants from the values measured at low energies to unifi- cation scale do not intercept at a single point while in the supersymmetric extension, the presence of additional particles, produces the convergence of coupling constants elegantly[4], or equivalently the prediction of the Wein- berg angle agrees with the experimental measurement very well[5]. This has become one of the cornerstone for believing the supersymmetric Standard 1 Model and the experimental search for the supersymmetry will be one of the main focus in the next round of new accelerators. In this paper we will explore the general possibilities of getting coupling constants unification by adding extra particles to the Standard Model[2] to see how unique is the Supersymmetric Standard Model in this respect[?]. -

The Quantum Mechanical Model of the Atom
The Quantum Mechanical Model of the Atom Quantum Numbers In order to describe the probable location of electrons, they are assigned four numbers called quantum numbers. The quantum numbers of an electron are kind of like the electron’s “address”. No two electrons can be described by the exact same four quantum numbers. This is called The Pauli Exclusion Principle. • Principle quantum number: The principle quantum number describes which orbit the electron is in and therefore how much energy the electron has. - it is symbolized by the letter n. - positive whole numbers are assigned (not including 0): n=1, n=2, n=3 , etc - the higher the number, the further the orbit from the nucleus - the higher the number, the more energy the electron has (this is sort of like Bohr’s energy levels) - the orbits (energy levels) are also called shells • Angular momentum (azimuthal) quantum number: The azimuthal quantum number describes the sublevels (subshells) that occur in each of the levels (shells) described above. - it is symbolized by the letter l - positive whole number values including 0 are assigned: l = 0, l = 1, l = 2, etc. - each number represents the shape of a subshell: l = 0, represents an s subshell l = 1, represents a p subshell l = 2, represents a d subshell l = 3, represents an f subshell - the higher the number, the more complex the shape of the subshell. The picture below shows the shape of the s and p subshells: (notice the electron clouds) • Magnetic quantum number: All of the subshells described above (except s) have more than one orientation. -

4 Nuclear Magnetic Resonance
Chapter 4, page 1 4 Nuclear Magnetic Resonance Pieter Zeeman observed in 1896 the splitting of optical spectral lines in the field of an electromagnet. Since then, the splitting of energy levels proportional to an external magnetic field has been called the "Zeeman effect". The "Zeeman resonance effect" causes magnetic resonances which are classified under radio frequency spectroscopy (rf spectroscopy). In these resonances, the transitions between two branches of a single energy level split in an external magnetic field are measured in the megahertz and gigahertz range. In 1944, Jevgeni Konstantinovitch Savoiski discovered electron paramagnetic resonance. Shortly thereafter in 1945, nuclear magnetic resonance was demonstrated almost simultaneously in Boston by Edward Mills Purcell and in Stanford by Felix Bloch. Nuclear magnetic resonance was sometimes called nuclear induction or paramagnetic nuclear resonance. It is generally abbreviated to NMR. So as not to scare prospective patients in medicine, reference to the "nuclear" character of NMR is dropped and the magnetic resonance based imaging systems (scanner) found in hospitals are simply referred to as "magnetic resonance imaging" (MRI). 4.1 The Nuclear Resonance Effect Many atomic nuclei have spin, characterized by the nuclear spin quantum number I. The absolute value of the spin angular momentum is L =+h II(1). (4.01) The component in the direction of an applied field is Lz = Iz h ≡ m h. (4.02) The external field is usually defined along the z-direction. The magnetic quantum number is symbolized by Iz or m and can have 2I +1 values: Iz ≡ m = −I, −I+1, ..., I−1, I. -

Critical Coupling for Dynamical Chiral-Symmetry Breaking with an Infrared Finite Gluon Propagator *
BR9838528 Instituto de Fisica Teorica IFT Universidade Estadual Paulista November/96 IFT-P.050/96 Critical coupling for dynamical chiral-symmetry breaking with an infrared finite gluon propagator * A. A. Natale and P. S. Rodrigues da Silva Instituto de Fisica Teorica Universidade Estadual Paulista Rua Pamplona 145 01405-900 - Sao Paulo, S.P. Brazil *To appear in Phys. Lett. B t 2 9-04 Critical Coupling for Dynamical Chiral-Symmetry Breaking with an Infrared Finite Gluon Propagator A. A. Natale l and P. S. Rodrigues da Silva 2 •r Instituto de Fisica Teorica, Universidade Estadual Paulista Rua Pamplona, 145, 01405-900, Sao Paulo, SP Brazil Abstract We compute the critical coupling constant for the dynamical chiral- symmetry breaking in a model of quantum chromodynamics, solving numer- ically the quark self-energy using infrared finite gluon propagators found as solutions of the Schwinger-Dyson equation for the gluon, and one gluon prop- agator determined in numerical lattice simulations. The gluon mass scale screens the force responsible for the chiral breaking, and the transition occurs only for a larger critical coupling constant than the one obtained with the perturbative propagator. The critical coupling shows a great sensibility to the gluon mass scale variation, as well as to the functional form of the gluon propagator. 'e-mail: [email protected] 2e-mail: [email protected] 1 Introduction The idea that quarks obtain effective masses as a result of a dynamical breakdown of chiral symmetry (DBCS) has received a great deal of attention in the last years [1, 2]. One of the most common methods used to study the quark mass generation is to look for solutions of the Schwinger-Dyson equation for the fermionic propagator. -

A Relativistic Electron in a Coulomb Potential
A Relativistic Electron in a Coulomb Potential Alfred Whitehead Physics 518, Fall 2009 The Problem Solve the Dirac Equation for an electron in a Coulomb potential. Identify the conserved quantum numbers. Specify the degeneracies. Compare with solutions of the Schrödinger equation including relativistic and spin corrections. Approach My approach follows that taken by Dirac in [1] closely. A few modifications taken from [2] and [3] are included, particularly in regards to the final quantum numbers chosen. The general strategy is to first find a set of transformations which turn the Hamiltonian for the system into a form that depends only on the radial variables r and pr. Once this form is found, I solve it to find the energy eigenvalues and then discuss the energy spectrum. The Radial Dirac Equation We begin with the electromagnetic Hamiltonian q H = p − cρ ~σ · ~p − A~ + ρ mc2 (1) 0 1 c 3 with 2 0 0 1 0 3 6 0 0 0 1 7 ρ1 = 6 7 (2) 4 1 0 0 0 5 0 1 0 0 2 1 0 0 0 3 6 0 1 0 0 7 ρ3 = 6 7 (3) 4 0 0 −1 0 5 0 0 0 −1 1 2 0 1 0 0 3 2 0 −i 0 0 3 2 1 0 0 0 3 6 1 0 0 0 7 6 i 0 0 0 7 6 0 −1 0 0 7 ~σ = 6 7 ; 6 7 ; 6 7 (4) 4 0 0 0 1 5 4 0 0 0 −i 5 4 0 0 1 0 5 0 0 1 0 0 0 i 0 0 0 0 −1 We note that, for the Coulomb potential, we can set (using cgs units): Ze2 p = −eΦ = − o r A~ = 0 This leads us to this form for the Hamiltonian: −Ze2 H = − − cρ ~σ · ~p + ρ mc2 (5) r 1 3 We need to get equation 5 into a form which depends not on ~p, but only on the radial variables r and pr. -
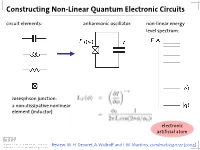
Constructing Non-Linear Quantum Electronic Circuits Circuit Elements: Anharmonic Oscillator: Non-Linear Energy Level Spectrum
Constructing Non-Linear Quantum Electronic Circuits circuit elements: anharmonic oscillator: non-linear energy level spectrum: Josesphson junction: a non-dissipative nonlinear element (inductor) electronic artificial atom Review: M. H. Devoret, A. Wallraff and J. M. Martinis, condmat/0411172 (2004) A Classification of Josephson Junction Based Qubits How to make use in of Jospehson junctions in a qubit? Common options of bias (control) circuits: phase qubit charge qubit flux qubit (Cooper Pair Box, Transmon) current bias charge bias flux bias How is the control circuit important? The Cooper Pair Box Qubit A Charge Qubit: The Cooper Pair Box discrete charge on island: continuous gate charge: total box capacitance Hamiltonian: electrostatic part: charging energy magnetic part: Josephson energy Hamilton Operator of the Cooper Pair Box Hamiltonian: commutation relation: charge number operator: eigenvalues, eigenfunctions completeness orthogonality phase basis: basis transformation Solving the Cooper Pair Box Hamiltonian Hamilton operator in the charge basis N : solutions in the charge basis: Hamilton operator in the phase basis δ : transformation of the number operator: solutions in the phase basis: Energy Levels energy level diagram for EJ=0: • energy bands are formed • bands are periodic in Ng energy bands for finite EJ • Josephson coupling lifts degeneracy • EJ scales level separation at charge degeneracy Charge and Phase Wave Functions (EJ << EC) courtesy CEA Saclay Charge and Phase Wave Functions (EJ ~ EC) courtesy CEA Saclay Tuning the Josephson Energy split Cooper pair box in perpendicular field = ( ) , cos cos 2 � � − − ̂ 0 SQUID modulation of Josephson energy consider two state approximation J. Clarke, Proc. IEEE 77, 1208 (1989) Two-State Approximation Restricting to a two-charge Hilbert space: Shnirman et al., Phys. -

1 Does Consciousness Really Collapse the Wave Function?
Does consciousness really collapse the wave function?: A possible objective biophysical resolution of the measurement problem Fred H. Thaheld* 99 Cable Circle #20 Folsom, Calif. 95630 USA Abstract An analysis has been performed of the theories and postulates advanced by von Neumann, London and Bauer, and Wigner, concerning the role that consciousness might play in the collapse of the wave function, which has become known as the measurement problem. This reveals that an error may have been made by them in the area of biology and its interface with quantum mechanics when they called for the reduction of any superposition states in the brain through the mind or consciousness. Many years later Wigner changed his mind to reflect a simpler and more realistic objective position, expanded upon by Shimony, which appears to offer a way to resolve this issue. The argument is therefore made that the wave function of any superposed photon state or states is always objectively changed within the complex architecture of the eye in a continuous linear process initially for most of the superposed photons, followed by a discontinuous nonlinear collapse process later for any remaining superposed photons, thereby guaranteeing that only final, measured information is presented to the brain, mind or consciousness. An experiment to be conducted in the near future may enable us to simultaneously resolve the measurement problem and also determine if the linear nature of quantum mechanics is violated by the perceptual process. Keywords: Consciousness; Euglena; Linear; Measurement problem; Nonlinear; Objective; Retina; Rhodopsin molecule; Subjective; Wave function collapse. * e-mail address: [email protected] 1 1. -

1.3 Running Coupling and Renormalization 27
1.3 Running coupling and renormalization 27 1.3 Running coupling and renormalization In our discussion so far we have bypassed the problem of renormalization entirely. The need for renormalization is related to the behavior of a theory at infinitely large energies or infinitesimally small distances. In practice it becomes visible in the perturbative expansion of Green functions. Take for example the tadpole diagram in '4 theory, d4k i ; (1.76) (2π)4 k2 m2 Z − which diverges for k . As we will see below, renormalizability means that the ! 1 coupling constant of the theory (or the coupling constants, if there are several of them) has zero or positive mass dimension: d 0. This can be intuitively understood as g ≥ follows: if M is the mass scale introduced by the coupling g, then each additional vertex in the perturbation series contributes a factor (M=Λ)dg , where Λ is the intrinsic energy scale of the theory and appears for dimensional reasons. If d 0, these diagrams will g ≥ be suppressed in the UV (Λ ). If it is negative, they will become more and more ! 1 relevant and we will find divergences with higher and higher orders.8 Renormalizability. The renormalizability of a quantum field theory can be deter- mined from dimensional arguments. Consider φp theory in d dimensions: 1 g S = ddx ' + m2 ' + 'p : (1.77) − 2 p! Z The action must be dimensionless, hence the Lagrangian has mass dimension d. From the kinetic term we read off the mass dimension of the field, namely (d 2)=2. The − mass dimension of 'p is thus p (d 2)=2, so that the dimension of the coupling constant − must be d = d + p pd=2.