Biogeochemical Cycle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Geochemical Cycle
Geochemical cycle In Earth science, a geochemical cycle is the pathway that chemical elements take in the surface and crust of the Earth.[1] The term "geochemical" tells us that geological and chemical factors are all included. The migration of heated and compressed chemical elements and compounds such as silicon, aluminium, and general alkali metals through the means of subduction and volcanism is known in the geological world as geochemical cycles. The geochemical cycle encompasses the natural separation and concentration of elements and heat-assisted recombination processes. Changes may not be apparent over a short term, such as with biogeochemical cycles, but over a long term changes of great magnitude occur, including the evolution of continents and oceans.[1] Contents Differentiating biogeochemical cycles Earth system Pathways Important cycles See also References Differentiating biogeochemical cycles Some may use the terms biogeochemical cycle and geochemical cycle interchangeably because both cycles deal with Earth's reservoirs. However, a biogeochemical cycle refers to the chemical interactions in surface reservoirs such as the atmosphere, hydrosphere, lithosphere, and biosphere whereas a geochemical cycle refers to the chemical interactions that exist in crustal and sub crustal reservoirs such as the deep earth and lithosphere. Earth system The Earth, as a system, is open to radiation from the sun and space, but is practically closed with regard to matter.[2] As all closed systems, it follows the law of conservation of mass which -

Earth Systems and Interactions
The Earth System Earth Systems and Interactions Key Concepts • How do Earth systems What do you think? Read the three statements below and decide interact in the carbon whether you agree or disagree with them. Place an A in the Before column cycle? if you agree with the statement or a D if you disagree. After you’ve read • How do Earth systems this lesson, reread the statements to see if you have changed your mind. interact in the phosphorus Before Statement After cycle? 1. The amount of water on Earth remains constant over time. 2. Hydrogen makes up the hydrosphere. 3. Most carbon on Earth is in the atmosphere. 3TUDY#OACH Earth Systems Make a Table Contrast the carbon cycle and the Your body contains many systems. These systems work phosphorus cycle in a two- together and make one big system—your body. Earth is a column table. Label one system, too. Like you, Earth has smaller systems that work column Carbon Cycle and together, or interact, and make the larger Earth system. Four the other column Phosphorus of these smaller systems are the atmosphere, the Cycle. Complete the table hydrosphere, the geosphere, and the biosphere. as you read this lesson. The Atmosphere Reading Check The outermost Earth system is a mixture of gases and 1. Identify What systems particles of matter called the atmosphere. It forms a layer make up the larger Earth around the other Earth systems. The atmosphere is mainly system? nitrogen and oxygen. Gases in the atmosphere move freely, helping transport matter and energy among Earth systems. -

Solar Thermochemical Hydrogen Production Research (STCH)
SANDIA REPORT SAND2011-3622 Unlimited Release Printed May 2011 Solar Thermochemical Hydrogen Production Research (STCH) Thermochemical Cycle Selection and Investment Priority Robert Perret Prepared by Sandia National Laboratories Albuquerque, New Mexico 87185 and Livermore, California 94550 Sandia National Laboratories is a multi-program laboratory managed and operated by Sandia Corporation, a wholly owned subsidiary of Lockheed Martin Corporation, for the U.S. Department of Energy’s National Nuclear Security Administration under contract DE-AC04-94AL85000. Approved for public release; further dissemination unlimited. Issued by Sandia National Laboratories, operated for the United States Department of Energy by Sandia Corporation. NOTICE: This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government, nor any agency thereof, nor any of their employees, nor any of their contractors, subcontractors, or their employees, make any warranty, express or implied, or assume any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represent that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government, any agency thereof, or any of their contractors or subcontractors. The views and opinions expressed herein do not necessarily state or reflect those of the United States Government, any agency thereof, or any of their contractors. Printed in the United States of America. This report has been reproduced directly from the best available copy. -

THE HYDROGEN ECONOMY. a Non-Technical Review
Hydrogen holds out the promise of a truly sustainable global energy future. As a clean energy carrier that can be produced from any primary energy source, hydrogen used in highly efficient fuel cells could prove to be the answer to our growing concerns about energy security, urban pollution and climate change. This prize surely warrants For more information, contact: THE HYDROGEN ECONOMY the attention and resources currently being UNEP DTIE directed at hydrogen – even if the Energy Branch prospects for widespread 39-43 Quai André Citroën commercialisation of hydrogen in the A non-technical review 75739 Paris Cedex 15, France foreseeable future are uncertain. Tel. : +33 1 44 37 14 50 Fax.: +33 1 44 37 14 74 E-mail: [email protected] www.unep.fr/energy/ ROGRAMME P NVIRONMENT E ATIONS N NITED DTI-0762-PA U Copyright © United Nations Environment Programme, 2006 This publication may be reproduced in whole or in part and in any form for educational or non-profit purposes without special permission from the copyright holder, provided acknowledgement of the source is made. UNEP would appreciate receiving a copy of any publication that uses this publication as a source. No use of this publication may be made for resale or for any other commercial purpose whatsoever without prior permission in writing from the United Nations Environment Programme. Disclaimer The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the United Nations Environment Programme concerning the legal status of any country, territory, city or area or of its authorities, or concerning delimitation of its frontiers or boundaries. -

Rock Cycle Roundabout
Rock Cycle Roundabout grade level 4th-8th; Standards for 4th and 7th subjects Earth & Space Science, Cause & Effect duration Prep Time: 20 min; Activity Time: 60 minutes, or two class periods setting Classroom Materials objectives - Rock Cycle Roundabout Board (1 per group) Students will be able to: - Rock Cycle Cards (1 set per 4 – 6 students) - small rocks, buttons, or other objects for game pieces 1. Differentiate among the three types of rock by (1 per student) referring to their methods of formation, providing - California Maps: Landforms, Waterways, and Faults real-world scenarios as examples. (1 per group, or projected for the class) 2. Recognize that some geologic processes are - Rock Types of California Map instantaneous, and others extremely gradual. (1 per group, or projected for the class) 3. Describe which processes might be affecting a - Student science notebooks or scratch paper given region, using evidence from natural features present on a map. scientific terms for students igneous rock: a type of rock that forms from the cooling and crust: the thin layer of solid rock that forms Earth’s outer hardening of magma or lava surface metamorphic rock: a type of rock that forms when a rock has mantle: the thick layer of hot, dense, rocky matter found below had its mineral composition and/or texture changed by heat the Earth’s crust and surrounding the Earth’s core and pressure magma: the molten material beneath or within the Earth’s sedimentary rock: a type of rock that forms when particles crust, from which igneous rock is formed from other rocks, or the remains of plants and animals, are lava: liquid magma that reaches the Earth’s surface pressed and cemented together weathering: the chemical and physical processes that break down rocks exposed to air, moisture, and organic matter at Background for Educators Earth’s surface The Earth, our rocky planet, is very active. -

HYDROGEN for HEATING: ATMOSPHERIC IMPACTS a Literature Review
HYDROGEN FOR HEATING: ATMOSPHERIC IMPACTS A literature review BEIS Research Paper Number 2018: no. 21 November 2018 7th October 2018 HYDROGEN FOR HEATING: ATMOSPHERIC IMPACTS – A LITERATURE REVIEW R.G. (Dick) Derwent OBE rdscientific, Newbury The preparation of this review and assessment was supported by the Department for Business, Energy and Industrial Strategy under Purchase Order No. 13070002913. BEIS Research Paper No. 21 1 HYDROGEN FOR HEATING: ATMOSPHERIC IMPACTS – A LITERATURE REVIEW Summary Introduction The Department for Business, Energy and Industrial Strategy (BEIS) is undertaking work to strengthen the evidence base on the potential long-term approaches for decarbonising heating. One approach being explored is whether hydrogen could be used in place of natural gas in the gas grid to provide a source of low-carbon heat in the future. BEIS commissioned rdscientific to carry out a literature review to assess the evidence on the potential atmospheric impacts of increased emissions to the atmosphere of hydrogen. Headline Findings This review summarises the present state of our understanding of the potential global atmospheric impacts of any future increased use of hydrogen through release of additional hydrogen into the atmosphere. This review has identified two global atmospheric dis- benefits from a future hydrogen economy: stratospheric ozone depletion through its moistening of the stratosphere, and contribution to climate change through increasing the growth rates of methane and tropospheric ozone. • The consensus from the limited number of studies using current stratospheric ozone models is that the impacts of hydrogen on the stratospheric ozone layer are small. • The best estimate of the carbon dioxide (CO2) equivalence of hydrogen is 4.3 megatonnes of carbon dioxide per 1 megatonne emission of hydrogen over a 100- year time horizon, the plausible range 0 – 9.8 expresses 95% confidence. -

The Rock Cycle
THE ROCK CYCLE Created by Kayla Rooney New Terms • Weathering: the various mechanical & chemical processes that cause exposed rock to decompose. • Lithification: the process by which materials are converted into solid rock through compaction or cementation. • Sediment: minerals or organic matter deposited by water, air or ice. • Metamorphism: a change in the structure of a rock due to natural processes such as, pressure or heat. • Crystallization: the act or process of crystallizing. • Solidification: a change from a liquid or gaseous state to a solid form. Sedimentary Rocks • Formed from pre-existing rocks or pieces of once- living organisms. • Formation occurs through the processes of deposition and lithification on Earth’s surface. • Distinct layering and bedding Examples of Sedimentary Rocks Igneous Rocks • Formed when hot, molten rock crystallizes and solidifies. • Found near active tectonic plate boundaries • Two types: 1) Intrusive (Plutonic): Formed when magma is trapped deep inside the Earth. 2) Extrusive (Volcanic): Formed when magma exits and cools above the Earth’s surface. Examples of Igneous Rocks Metamorphic Rocks • Formed from other types of rocks (sedimentary, igneous or other metamorphic rocks) • Formed from the processes of high heat and high pressure deep within the Earth. • The final result is a more dense and compact rock with a new mineral make up. Examples of Metamorphic Rocks References • https://geomaps.wr.usgs.gov/parks/rxmin/rock2.html • https://www.usgs.gov/faqs/what-are-igneous-rocks?qt-news_science_products=0#qt-news_science_products -

The Rock Cycle
The Rock Cycle There are three major classifications of rock, based on the method of their formation: igneous rock, metamorphic rock, and sedimentary rock. The rock cycle is the series of processes by which rocks are transformed from one type to another and continually renewed. The origin of all rock can be ultimately traced back to the solidification of molten magma. Magma is a hot liquid made of melted minerals and compounds commonly found in rocks. The rock cycle is a model that describes how rocks are created, changed, and destroyed. There are three major types of rock: igneous rock, metamorphic rock, and sedimentary rock. During the rock cycle, each type of rock may be changed into another type. The rock cycle also includes several different processes. Crystallization is the process by which magma cools and forms solid rock. Heat and pressure often change one type of rock into another. Weathering, erosion, and deposition are the processes that break rock down into sediment at the Earth's surface. Wind, rain, running water, and ice commonly take part in these processes. Compaction and cementation—also known as lithification—is the process of loose sediments being formed into sedimentary rocks. And melting, of course, is the process that transforms solid rock back into liquid magma. The rock cycle is a process that takes hundreds of millions of years. But since it has operated continuously during Earth's history, new rock at the Earth's surface is constantly replacing old rock. Igneous Rock Igneous rock forms when magma and lava cool and make mineral crystals. -
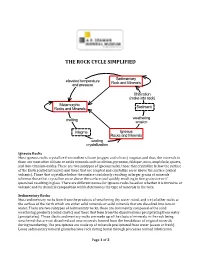
The Rock Cycle Simplified
THE ROCK CYCLE SIMPLIFIED Sedimentary elevated temperature Rock and Minerals and pressure lithification (make into rock) Metamorphic Rocks and Minerals Sediment melting weathering erosion Magma Igneous Rocks and Minerals cooling crystallization Igneous Rocks Most igneous rocks crystallize from molten silicate (oxygen and silicon) magmas and thus, the minerals in them are most often silicate or oxide minerals such as olivine, pyroxene, feldspar, mica, amphibole, quartz, and iron-titanium oxides. There are two subtypes of igneous rocks, those that crystallize below the surface of the Earth (called intrusive) and those that are erupted and crystallize on or above the surface (called volcanic). Those that crystallize below the surface cool slowly resulting in larger grains of minerals whereas those that crystallize on or above the surface cool quickly resulting in fine grain size or if quenched resulting in glass. There are different names for igneous rocks based on whether it is intrusive or volcanic and its chemical composition which determines the type of minerals in the rock. Sedimentary Rocks Most sedimentary rocks form from the products of weathering (by water, wind, and ice) of other rocks at the surface of the Earth which are either solid minerals or solid minerals that are dissolved into ions in water. There are two subtypes of sedimentary rocks, those are dominantly composed of the solid weathering products (called clastic) and those that form from the dissolved ions precipitating from water (precipitates). Those clastic sedimentary rocks are made up of the clasts of minerals in the rock being weathered that are not dissolved and new minerals formed from the breakdown of original minerals during weathering. -

Rock Cycle Geol
THE ROCK CYCLE THE ROCK CYCLE www.geolsoc.org/factsheets www.geolsoc.org/factsheets YOU WILL NEED: There are three main types of rocks: igneous, sedimentary and metamorphic ñ Geological Society ‘The Rock Cycle' rocks and these rocks all form in different ways. Fill in the questions below factsheet 2. SEDIMENTARY ROCKS test your rock knowledge. ñ Colouring pencils a) Use the space below to draw the stages in which a sedimentary rock might form. ñ Basalt and granite rock samples (optional) 1. THE ROCK CYCLE WEATHERING: wind, rain & ice EROSION: rivers, glaciers & wind DEPOSITION: sediments are laid Use the phrases in the word bank below to label the different processes and rock types in the rock cycle. break up rock into fragments transport the sediment away down in layers in a sea or lake WORD Metamorphosis Erosion & transport Metamorphic rock Crystallisation of Weathering Melting Uplift magma Sedimentary rock BANK: Burial & compaction Igneous rock Deposition of sediments Geolsoc.org/factsheets BURIAL & COMPACTION: SEDIMENTARY ROCK: sediments are pushed deeper and sediments are cemented into a rock compacted over time Basalt Granite 3. IGNEOUS ROCKS Granite and basalt are two types of igneous rock. Granite has large crystals whereas basalt has tiny crystals. a) Label the diagram to show where each rock forms. b) Why do you think granite usually has larger crystals than basalt? Tip: Think about temperature. ______________________________________________________________________ ______________________________________________________________________ _____________________________________________________________________ 4. METAMORPHIC ROCKS How can a sedimentary rock become a metamorphic rock? ___________________________________________________________________________________________________________________________________________ ___________________________________________________________________________________________________________________________________________ . -

Biogeochemical Cycle
Biogeochemical cycle In ecology and Earth science, a biogeochemical cycle or substance turnover or cycling of substances is a pathway by which a chemical substance moves through biotic (biosphere) and abiotic (lithosphere, atmosphere, and hydrosphere) compartments of Earth. There are biogeochemical cycles for the chemical elements calcium, carbon, hydrogen, mercury, nitrogen, oxygen, phosphorus, selenium, and sulfur; molecular cycles for water and silica; macroscopic cycles such as the rock cycle; as well as human-induced cycles for synthetic compounds such as An illustration of the oceanic whale pump polychlorinated biphenyl (PCB). In some showing how whales cycle nutrients cycles there are reservoirs where a through the water column substance remains for a long period of time. Contents Systems Reservoirs Important cycles See also References Further reading Systems Ecological systems (ecosystems) have many biogeochemical cycles operating as a part of the system, for example the water cycle, the carbon cycle, the nitrogen cycle, etc. All chemical elements occurring in organisms are part of biogeochemical cycles. In addition to being a part of living organisms, these chemical elements also cycle through abiotic factors of ecosystems such as water (hydrosphere), land (lithosphere), and/or the air (atmosphere).[1] The living factors of the planet can be referred to collectively as the biosphere. All the nutrients—such as carbon, nitrogen, oxygen, phosphorus, and sulfur—used in ecosystems by living organisms are a part of a closed system; therefore, these chemicals are recycled instead of being lost and replenished constantly such as in an open system.[1] The flow of energy in an ecosystem is an open system; the sun constantly gives the planet energy in the form of light while it is eventually used and lost in the form of heat throughout the trophic levels of a food web. -

Types of Rock and the Rock Cycle
TEACHER BACKGROUND KNOWEDGE Minerals, Rocks and the Rock Cycle Core Concepts • Rocks in the Earth’s crust vary in their form and structure based on process that made them. The constant changing of the form and structure of rocks is called the rock cycle. The energy powering the rock cycle comes from the sun and the earth’s core. o Minerals o Igneous Rock o Metamorphic Rock o Sedimentary Rock o The Rock Cycle Minerals The study of minerals is important because all rocks are made of minerals. A mineral is a solid substance that occurs in nature, is inorganic, and has a specific chemical composition. Examples of minerals are diamonds, quartz, and table salt. Minerals are NSF/IERI Science IDEAS #0228353 crystalline, meaning that they are made up of atoms in specific, orderly, repeating structure. Rocks can be broken down mechanically into their individual minerals. Minerals themselves cannot be mechanically divided down into any of their constituent parts. The two main factors that differentiate one type of mineral from another are the chemical composition and the crystalline arrangement of the atoms. Chemical composition refers to the type and proportion of atoms that make up the mineral. For example, table salt is made up of two kinds of atoms (sodium and chlorine) in equal proportion (one sodium atom for each chlorine atom as shown by the formula NaCl). Quartz is made up of a combination of one silicon atom for every two oxygen atoms and is shown by the formula SiO2. A different ratio of materials would result in a different kind of mineral.