Sensory Ecology of Spiders That Prey on Frogs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CAT Vertebradosgt CDC CECON USAC 2019
Catálogo de Autoridades Taxonómicas de vertebrados de Guatemala CDC-CECON-USAC 2019 Centro de Datos para la Conservación (CDC) Centro de Estudios Conservacionistas (Cecon) Facultad de Ciencias Químicas y Farmacia Universidad de San Carlos de Guatemala Este documento fue elaborado por el Centro de Datos para la Conservación (CDC) del Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala. Guatemala, 2019 Textos y edición: Manolo J. García. Zoólogo CDC Primera edición, 2019 Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala ISBN: 978-9929-570-19-1 Cita sugerida: Centro de Estudios Conservacionistas [Cecon]. (2019). Catálogo de autoridades taxonómicas de vertebrados de Guatemala (Documento técnico). Guatemala: Centro de Datos para la Conservación [CDC], Centro de Estudios Conservacionistas [Cecon], Facultad de Ciencias Químicas y Farmacia, Universidad de San Carlos de Guatemala [Usac]. Índice 1. Presentación ............................................................................................ 4 2. Directrices generales para uso del CAT .............................................. 5 2.1 El grupo objetivo ..................................................................... 5 2.2 Categorías taxonómicas ......................................................... 5 2.3 Nombre de autoridades .......................................................... 5 2.4 Estatus taxonómico -

Xenosaurus Tzacualtipantecus. the Zacualtipán Knob-Scaled Lizard Is Endemic to the Sierra Madre Oriental of Eastern Mexico
Xenosaurus tzacualtipantecus. The Zacualtipán knob-scaled lizard is endemic to the Sierra Madre Oriental of eastern Mexico. This medium-large lizard (female holotype measures 188 mm in total length) is known only from the vicinity of the type locality in eastern Hidalgo, at an elevation of 1,900 m in pine-oak forest, and a nearby locality at 2,000 m in northern Veracruz (Woolrich- Piña and Smith 2012). Xenosaurus tzacualtipantecus is thought to belong to the northern clade of the genus, which also contains X. newmanorum and X. platyceps (Bhullar 2011). As with its congeners, X. tzacualtipantecus is an inhabitant of crevices in limestone rocks. This species consumes beetles and lepidopteran larvae and gives birth to living young. The habitat of this lizard in the vicinity of the type locality is being deforested, and people in nearby towns have created an open garbage dump in this area. We determined its EVS as 17, in the middle of the high vulnerability category (see text for explanation), and its status by the IUCN and SEMAR- NAT presently are undetermined. This newly described endemic species is one of nine known species in the monogeneric family Xenosauridae, which is endemic to northern Mesoamerica (Mexico from Tamaulipas to Chiapas and into the montane portions of Alta Verapaz, Guatemala). All but one of these nine species is endemic to Mexico. Photo by Christian Berriozabal-Islas. amphibian-reptile-conservation.org 01 June 2013 | Volume 7 | Number 1 | e61 Copyright: © 2013 Wilson et al. This is an open-access article distributed under the terms of the Creative Com- mons Attribution–NonCommercial–NoDerivs 3.0 Unported License, which permits unrestricted use for non-com- Amphibian & Reptile Conservation 7(1): 1–47. -

Dna Sequence Data Indicates the Polyphyl Y of the Family Ctenidae (Araneae )
1993. The Journal of Arachnology 21 :194–201 DNA SEQUENCE DATA INDICATES THE POLYPHYL Y OF THE FAMILY CTENIDAE (ARANEAE ) Kathrin C . Huber', Thomas S . Haider2, Manfred W . Miiller2, Bernhard A . Huber' , Rudolf J. Schweyen2, and Friedrich G . Barth' : 'Institut fair Zoologie, Althanstr . 14; 1090 Wien; and 2lnstitut fur Mikrobiologie and Genetik; Dr. Bohrgasse 9 ; 1030 Wien (Vienna), Austria . ABSTRACT. Mitochondrial DNA fragments comprising more than 400 bases of the 16S rDNA from nine spider species have been sequenced: Cupiennius salei, C. getazi, C. coccineus and Phoneutria boliviensis (Ctenidae), Pisaura mirabilis, Dolomedes fimbriatus (Pisauridae), Pardosa agrestis (Lycosidae), Clubiona pallidula (Clubi- onidae) and Ryuthela nishihirai (syn. Heptathela nishihirai; Heptathelidae: Mesothelae). Sequence divergence ranges from 3–4% among Cupiennius species and up to 36% in pairwise comparisons of the more distantly related spider DNAs. Maximally parsimonious gene trees based on these sequences indicate that Phoneutri a and Cupiennius are the most distantly related species of the examined Lycosoidea . The monophyly of the family Ctenidae is therefore doubted ; and a revision of the family, which should include DNA-data, is needed . Cupiennius salei (Ctenidae) is one of the most get a high copy number of the DNA segment of extensively studied species of spiders (see Lach - interest. The PCR depends on the availability of muth et al. 1985). The phylogeny of the Ctenidae , oligonucleotides that specifically bind to the a mainly South and Central American family, i s flanking sequences of this DNA segment. These poorly understood ; and systematists propose oligonucleotides serve as primers for a polymer- highly contradicting views on its classification ization reaction that copies the segment in vitro. -

SPIDERS of WASHINGTON COUNTY, MISSISSIPPI Orrey P. Young Southern Field Crop Insect Management Laboratory USDA-ARS, P.O. Box
Young, O . P., T. C . Lockley and G . B . Edwards . 1989 . Spiders of Washington County, Mississippi . J . Arachnol ., 17 :27-41 . SPIDERS OF WASHINGTON COUNTY, MISSISSIPPI Orrey P. Young Southern Field Crop Insect Management Laboratory USDA-ARS, P.O. Box 346 Stoneville, Mississippi 38776 USA Timothy C. Lockley Imported Fire Ant Station USDA-APHIS-PPQ 3505 25th Avenue Gulfport, Mississippi 39501 USA and G. B. Edwards Florida State Collection of Arthropods Division of Plant Industry Florida Dept. Agric. & Cons . Serv. P.O. Box 1269 Gainesville, Florida 32602 USA ABSTRACT Over a seven-year period, approximately 35,000 spiders representing 26 families, 133 genera, and 234 species were captured in Washington County, Mississippi, by pitfall, sweepnet, vacuum, bag, and hand. Specimens were collected in 10 different habitat types and in four vegetational strata . Old-field habitats yielded the most species (152) and residential lawns the fewest (14) . Considering all habitats sampled, the ground layer produced 111 species, the herbaceous strata 133, the shrub layer 49, and the tree strata 30 species . The sweepnet method of capture obtained 128 species, pitfall 95, hand 61, vacuum 53, and bagging 19 species. The largest number of species were obtained in spring and early summer (maximum of 125 in May), with the fewest in mid-winter (Jan . = 24) . Twenty-one species were considered abundant, 51 common, 67 uncommon, and 95 rare . Additions to the state list of Dorris (1972) number 102 species, for a new state total of 364 species . A comparison with the North American fauna and with other surveys indicates that Washington County is underrepresented both in cursorial forms active on the soil surface and web-spinning forms typical of undisturbed habitats . -

Abundance and Community Composition of Arboreal Spiders: the Relative Importance of Habitat Structure
AN ABSTRACT OF THE THESIS OF Juraj Halaj for the degree of Doctor of Philosophy in Entomology presented on May 6, 1996. Title: Abundance and Community Composition of Arboreal Spiders: The Relative Importance of Habitat Structure. Prey Availability and Competition. Abstract approved: Redacted for Privacy _ John D. Lattin, Darrell W. Ross This work examined the importance of structural complexity of habitat, availability of prey, and competition with ants as factors influencing the abundance and community composition of arboreal spiders in western Oregon. In 1993, I compared the spider communities of several host-tree species which have different branch structure. I also assessed the importance of several habitat variables as predictors of spider abundance and diversity on and among individual tree species. The greatest abundance and species richness of spiders per 1-m-long branch tips were found on structurally more complex tree species, including Douglas-fir, Pseudotsuga menziesii (Mirbel) Franco and noble fir, Abies procera Rehder. Spider densities, species richness and diversity positively correlated with the amount of foliage, branch twigs and prey densities on individual tree species. The amount of branch twigs alone explained almost 70% of the variation in the total spider abundance across five tree species. In 1994, I experimentally tested the importance of needle density and branching complexity of Douglas-fir branches on the abundance and community structure of spiders and their potential prey organisms. This was accomplished by either removing needles, by thinning branches or by tying branches. Tying branches resulted in a significant increase in the abundance of spiders and their prey. Densities of spiders and their prey were reduced by removal of needles and thinning. -

Zootaxa, Anura, Leptodactylidae
Zootaxa 1116: 43–54 (2006) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA 1116 Copyright © 2006 Magnolia Press ISSN 1175-5334 (online edition) Reassessment of the taxonomic status of the genera Ischnocnema Reinhardt and Lütken, 1862 and Oreobates Jiménez-de-la-Espada, 1872, with notes on the synonymy of Leiuperus verrucosus Reinhardt and Lütken, 1862 (Anura: Leptodactylidae) ULISSES CARAMASCHI & CLARISSA CANEDO Departamento de Vertebrados, Museu Nacional/UFRJ, Quinta da Boa Vista, 20940-040 Rio de Janeiro, RJ, Brasil ([email protected], [email protected]). Abstract The taxonomic status of two leptodactylid frog genera is reevaluated. Ischnocnema Reinhardt and Lütken, 1862 is considered a junior synonym of Eleutherodactylus Duméril and Bibron, and the combination E. verrucosus (Reinhardt and Lütken, 1862) is proposed. Oreobates Jiménez-de-la- Espada, 1872 is revalidated, and the combinations Oreobates quixensis Jiménez-de-la-Espada, 1872, O. simmonsi (Lynch, 1974), O. saxatilis (Duellman, 1990), O. sanctaecrucis (Harvey and Keck, 1995), and O. sanderi (Padial, Reichle and De la Riva, 2005) are proposed. Epsophus verrucosus Miranda-Ribeiro, 1937 is synonymized with Eleutherodactylus verrucosus (Reinhardt and Lütken, 1862). Key words: Amphibia; Anura; Leptodactylidae; Ischnocnema; Oreobates; Taxonomy Introduction WIDELY disjunct geographical distributions among closely related species are rare in the Neotropics. In general, a more detailed analysis reveals that these species are, in reality, members of distinct evolutionary lineages. The geographical distribution of the species currently placed in the genus Ischnocnema Reinhardt and Lütken, 1862 presents such a gap. One species, I. verrucosa (Reinhardt and Lütken, 1862), occurs in Southeastern Brazil, and the other five, I. -

Western Ghats & Sri Lanka Biodiversity Hotspot
Ecosystem Profile WESTERN GHATS & SRI LANKA BIODIVERSITY HOTSPOT WESTERN GHATS REGION FINAL VERSION MAY 2007 Prepared by: Kamal S. Bawa, Arundhati Das and Jagdish Krishnaswamy (Ashoka Trust for Research in Ecology & the Environment - ATREE) K. Ullas Karanth, N. Samba Kumar and Madhu Rao (Wildlife Conservation Society) in collaboration with: Praveen Bhargav, Wildlife First K.N. Ganeshaiah, University of Agricultural Sciences Srinivas V., Foundation for Ecological Research, Advocacy and Learning incorporating contributions from: Narayani Barve, ATREE Sham Davande, ATREE Balanchandra Hegde, Sahyadri Wildlife and Forest Conservation Trust N.M. Ishwar, Wildlife Institute of India Zafar-ul Islam, Indian Bird Conservation Network Niren Jain, Kudremukh Wildlife Foundation Jayant Kulkarni, Envirosearch S. Lele, Centre for Interdisciplinary Studies in Environment & Development M.D. Madhusudan, Nature Conservation Foundation Nandita Mahadev, University of Agricultural Sciences Kiran M.C., ATREE Prachi Mehta, Envirosearch Divya Mudappa, Nature Conservation Foundation Seema Purshothaman, ATREE Roopali Raghavan, ATREE T. R. Shankar Raman, Nature Conservation Foundation Sharmishta Sarkar, ATREE Mohammed Irfan Ullah, ATREE and with the technical support of: Conservation International-Center for Applied Biodiversity Science Assisted by the following experts and contributors: Rauf Ali Gladwin Joseph Uma Shaanker Rene Borges R. Kannan B. Siddharthan Jake Brunner Ajith Kumar C.S. Silori ii Milind Bunyan M.S.R. Murthy Mewa Singh Ravi Chellam Venkat Narayana H. Sudarshan B.A. Daniel T.S. Nayar R. Sukumar Ranjit Daniels Rohan Pethiyagoda R. Vasudeva Soubadra Devy Narendra Prasad K. Vasudevan P. Dharma Rajan M.K. Prasad Muthu Velautham P.S. Easa Asad Rahmani Arun Venkatraman Madhav Gadgil S.N. Rai Siddharth Yadav T. Ganesh Pratim Roy Santosh George P.S. -
For Review Only
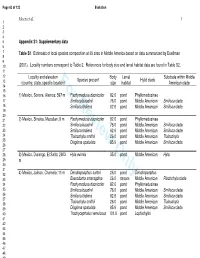
Page 63 of 123 Evolution Moen et al. 1 1 2 3 4 5 Appendix S1: Supplementary data 6 7 Table S1 . Estimates of local species composition at 39 sites in Middle America based on data summarized by Duellman 8 9 10 (2001). Locality numbers correspond to Table 2. References for body size and larval habitat data are found in Table S2. 11 12 Locality and elevation Body Larval Subclade within Middle Species present Hylid clade 13 (country, state, specific location)For Reviewsize Only habitat American clade 14 15 16 1) Mexico, Sonora, Alamos; 597 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 17 Smilisca baudinii 76.0 pond Middle American Smilisca clade 18 Smilisca fodiens 62.6 pond Middle American Smilisca clade 19 20 21 2) Mexico, Sinaloa, Mazatlan; 9 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 22 Smilisca baudinii 76.0 pond Middle American Smilisca clade 23 Smilisca fodiens 62.6 pond Middle American Smilisca clade 24 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 25 Diaglena spatulata 85.9 pond Middle American Smilisca clade 26 27 28 3) Mexico, Durango, El Salto; 2603 Hyla eximia 35.0 pond Middle American Hyla 29 m 30 31 32 4) Mexico, Jalisco, Chamela; 11 m Dendropsophus sartori 26.0 pond Dendropsophus 33 Exerodonta smaragdina 26.0 stream Middle American Plectrohyla clade 34 Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 35 Smilisca baudinii 76.0 pond Middle American Smilisca clade 36 Smilisca fodiens 62.6 pond Middle American Smilisca clade 37 38 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 39 Diaglena spatulata 85.9 pond Middle American Smilisca clade 40 Trachycephalus venulosus 101.0 pond Lophiohylini 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 Evolution Page 64 of 123 Moen et al. -

Mannophryne Olmonae) Catherine G
The College of Wooster Libraries Open Works Senior Independent Study Theses 2014 A Not-So-Silent Spring: The mpI acts of Traffic Noise on Call Features of The loB ody Bay Poison Frog (Mannophryne olmonae) Catherine G. Clemmens The College of Wooster, [email protected] Follow this and additional works at: https://openworks.wooster.edu/independentstudy Part of the Other Environmental Sciences Commons Recommended Citation Clemmens, Catherine G., "A Not-So-Silent Spring: The mpI acts of Traffico N ise on Call Features of The loodyB Bay Poison Frog (Mannophryne olmonae)" (2014). Senior Independent Study Theses. Paper 5783. https://openworks.wooster.edu/independentstudy/5783 This Senior Independent Study Thesis Exemplar is brought to you by Open Works, a service of The oC llege of Wooster Libraries. It has been accepted for inclusion in Senior Independent Study Theses by an authorized administrator of Open Works. For more information, please contact [email protected]. © Copyright 2014 Catherine G. Clemmens A NOT-SO-SILENT SPRING: THE IMPACTS OF TRAFFIC NOISE ON CALL FEATURES OF THE BLOODY BAY POISON FROG (MANNOPHRYNE OLMONAE) DEPARTMENT OF BIOLOGY INDEPENDENT STUDY THESIS Catherine Grace Clemmens Adviser: Richard Lehtinen Submitted in Partial Fulfillment of the Requirement for Independent Study Thesis in Biology at the COLLEGE OF WOOSTER 2014 TABLE OF CONTENTS I. ABSTRACT II. INTRODUCTION…………………………………………...............…...........1 a. Behavioral Effects of Anthropogenic Noise……………………….........2 b. Effects of Anthropogenic Noise on Frog Vocalization………………....6 c. Why Should We Care? The Importance of Calling for Frogs..................8 d. Color as a Mode of Communication……………………………….…..11 e. Biology of the Bloody Bay Poison Frog (Mannophryne olmonae)…...13 III. -

Species Diversity and Conservation Status of Amphibians in Madre De Dios, Southern Peru
Herpetological Conservation and Biology 4(1):14-29 Submitted: 18 December 2007; Accepted: 4 August 2008 SPECIES DIVERSITY AND CONSERVATION STATUS OF AMPHIBIANS IN MADRE DE DIOS, SOUTHERN PERU 1,2 3 4,5 RUDOLF VON MAY , KAREN SIU-TING , JENNIFER M. JACOBS , MARGARITA MEDINA- 3 6 3,7 1 MÜLLER , GIUSEPPE GAGLIARDI , LILY O. RODRÍGUEZ , AND MAUREEN A. DONNELLY 1 Department of Biological Sciences, Florida International University, 11200 SW 8th Street, OE-167, Miami, Florida 33199, USA 2 Corresponding author, e-mail: [email protected] 3 Departamento de Herpetología, Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Avenida Arenales 1256, Lima 11, Perú 4 Department of Biology, San Francisco State University, 1600 Holloway Avenue, San Francisco, California 94132, USA 5 Department of Entomology, California Academy of Sciences, 55 Music Concourse Drive, San Francisco, California 94118, USA 6 Departamento de Herpetología, Museo de Zoología de la Universidad Nacional de la Amazonía Peruana, Pebas 5ta cuadra, Iquitos, Perú 7 Programa de Desarrollo Rural Sostenible, Cooperación Técnica Alemana – GTZ, Calle Diecisiete 355, Lima 27, Perú ABSTRACT.—This study focuses on amphibian species diversity in the lowland Amazonian rainforest of southern Peru, and on the importance of protected and non-protected areas for maintaining amphibian assemblages in this region. We compared species lists from nine sites in the Madre de Dios region, five of which are in nationally recognized protected areas and four are outside the country’s protected area system. Los Amigos, occurring outside the protected area system, is the most species-rich locality included in our comparison. -

Bibliography and Scientific Name Index to Amphibians
lb BIBLIOGRAPHY AND SCIENTIFIC NAME INDEX TO AMPHIBIANS AND REPTILES IN THE PUBLICATIONS OF THE BIOLOGICAL SOCIETY OF WASHINGTON BULLETIN 1-8, 1918-1988 AND PROCEEDINGS 1-100, 1882-1987 fi pp ERNEST A. LINER Houma, Louisiana SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE NO. 92 1992 SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE The SHIS series publishes and distributes translations, bibliographies, indices, and similar items judged useful to individuals interested in the biology of amphibians and reptiles, but unlikely to be published in the normal technical journals. Single copies are distributed free to interested individuals. Libraries, herpetological associations, and research laboratories are invited to exchange their publications with the Division of Amphibians and Reptiles. We wish to encourage individuals to share their bibliographies, translations, etc. with other herpetologists through the SHIS series. If you have such items please contact George Zug for instructions on preparation and submission. Contributors receive 50 free copies. Please address all requests for copies and inquiries to George Zug, Division of Amphibians and Reptiles, National Museum of Natural History, Smithsonian Institution, Washington DC 20560 USA. Please include a self-addressed mailing label with requests. INTRODUCTION The present alphabetical listing by author (s) covers all papers bearing on herpetology that have appeared in Volume 1-100, 1882-1987, of the Proceedings of the Biological Society of Washington and the four numbers of the Bulletin series concerning reference to amphibians and reptiles. From Volume 1 through 82 (in part) , the articles were issued as separates with only the volume number, page numbers and year printed on each. Articles in Volume 82 (in part) through 89 were issued with volume number, article number, page numbers and year. -

Reflections on the Tapetum Lucidum and Eyeshine in Lycosoid Spiders
2013. The Journal of Arachnology 41:43–52 Reflections on the tapetum lucidum and eyeshine in lycosoid spiders Kari Benson: School of Sciences, Lynchburg College, 1501 Lakeside Dr., Lynchburg, Virginia 24501, USA. E-mail: [email protected] Robert B. Suter: Department of Biology, Vassar College, 124 Raymond Avenue, Poughkeepsie, New York 12603, USA Abstract. In the lycosoid spiders, the secondary eyes possess a grate-shaped tapetum lucidum that reflects light, causing eyeshine when these spiders are viewed with approximately coaxial illumination. This guanine-based reflective surface is thought to increase visual capabilities in low light. We explored the eyeshine of the posterior medial eye in eight taxa of pisaurid and lycosid spiders. The taxa included four pisaurids: Dolomedes tenebrosus Hentz 1844, D. triton (Walckenaer 1837), D. scriptus Hentz 1845 and D. vittatus Walckenaer 1837; and four lycosids: Gladicosa pulchra (Keyserling 1877), Hogna sp. (cf. Lycosa lenta (Hentz 1844) sensu Wallace 1942), Rabidosa punctulata (Hentz 1844) and Varacosa avara (Keyserling 1877). We found that there were significant family- and species-level differences in both the reflected spectra and the intensity of reflection. Although the peaks of the reflected spectra were in the green range for all spiders, the mean peak was further toward the blue end of the spectrum for the lycosids than for the pisaurids. Variation among species (about 54% of the total variation) was dominated by G. pulchra (Lycosidae) and D. vittatus (Pisauridae), both of whose spectra peaked near yellow, vs. V. avara (Lycosidae), whose spectra peaked to the blue side of green. The lycosid spiders showed overall brighter eyeshine.