Introduction Central America Has One of the Most Diverse Moss Floras in the World. Its 871 Species Are Found in an Area Three-F
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
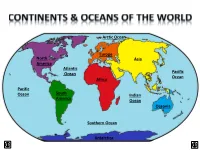
North America Other Continents
Arctic Ocean Europe North Asia America Atlantic Ocean Pacific Ocean Africa Pacific Ocean South Indian America Ocean Oceania Southern Ocean Antarctica LAND & WATER • The surface of the Earth is covered by approximately 71% water and 29% land. • It contains 7 continents and 5 oceans. Land Water EARTH’S HEMISPHERES • The planet Earth can be divided into four different sections or hemispheres. The Equator is an imaginary horizontal line (latitude) that divides the earth into the Northern and Southern hemispheres, while the Prime Meridian is the imaginary vertical line (longitude) that divides the earth into the Eastern and Western hemispheres. • North America, Earth’s 3rd largest continent, includes 23 countries. It contains Bermuda, Canada, Mexico, the United States of America, all Caribbean and Central America countries, as well as Greenland, which is the world’s largest island. North West East LOCATION South • The continent of North America is located in both the Northern and Western hemispheres. It is surrounded by the Arctic Ocean in the north, by the Atlantic Ocean in the east, and by the Pacific Ocean in the west. • It measures 24,256,000 sq. km and takes up a little more than 16% of the land on Earth. North America 16% Other Continents 84% • North America has an approximate population of almost 529 million people, which is about 8% of the World’s total population. 92% 8% North America Other Continents • The Atlantic Ocean is the second largest of Earth’s Oceans. It covers about 15% of the Earth’s total surface area and approximately 21% of its water surface area. -

New Data on the Moss Genus Hymenoloma (Bryophyta), with Special Reference to H
Cryptogamie, Bryologie, 2013, 34 (1): 1-18 © 2013 Adac. Tous droits réservés New data on the moss genus Hymenoloma (Bryophyta), with special reference to H. mulahaceni Olaf WERNER a, Susana RAMS b, Jan KUČERA c, Juan LARRAÍN d, Olga M. AFONINA e, Sergio PISA a & Rosa María ROS a* aDepartamento de Biología Vegetal (Botánica), Universidad de Murcia, Campus de Espinardo, E-30100 Murcia, Spain bEscuela Universitaria de Magisterio “La Inmaculada”, Universidad de Granada, Carretera de Murcia s/n, 18010 Granada, Spain cUniversity of South Bohemia, Faculty of Science, Branišovská 31, CZ - 370 05 České Budějovice, Czech Republic dUniversidad de Concepción, Departamento de Botánica, Casilla 160-C, Concepción, Chile eKomarov Botanical Institute of Russian Academy of Sciences, Professor Popov Str. 2, St.-Petersburg 197376, Russia Abstract – A molecular and morphological study using two chloroplast molecular markers (rps4 and trnL-F) was carried out with specimens belonging to Hymenoloma mulahaceni, a species described at the end of the 19th century from the Sierra Nevada Mountains in southern Spain as a member of Oreoweisia. The comparison with Asian, European, and North American material of Dicranoweisia intermedia proved the conspecifity of both taxa, which was corroborated by molecular data. Therefore, the distribution area of H. mulahaceni is extended to U.S.A., Canada, Greenland, and several Asian countries (Armenia, Georgia, Tajikistan, and Uzbekistan). We also tested the monophyly of Hymenoloma sensu Ochyra et al. (2003), by including in the analysis the Holarctic taxa assigned to the genus together with Chilean material identified as H. antarcticum (putatively synonymous with the type of Hymenoloma) and H. -

Natural Heritage Program List of Rare Plant Species of North Carolina 2016
Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Revised February 24, 2017 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org C ur Alleghany rit Ashe Northampton Gates C uc Surry am k Stokes P d Rockingham Caswell Person Vance Warren a e P s n Hertford e qu Chowan r Granville q ot ui a Mountains Watauga Halifax m nk an Wilkes Yadkin s Mitchell Avery Forsyth Orange Guilford Franklin Bertie Alamance Durham Nash Yancey Alexander Madison Caldwell Davie Edgecombe Washington Tyrrell Iredell Martin Dare Burke Davidson Wake McDowell Randolph Chatham Wilson Buncombe Catawba Rowan Beaufort Haywood Pitt Swain Hyde Lee Lincoln Greene Rutherford Johnston Graham Henderson Jackson Cabarrus Montgomery Harnett Cleveland Wayne Polk Gaston Stanly Cherokee Macon Transylvania Lenoir Mecklenburg Moore Clay Pamlico Hoke Union d Cumberland Jones Anson on Sampson hm Duplin ic Craven Piedmont R nd tla Onslow Carteret co S Robeson Bladen Pender Sandhills Columbus New Hanover Tidewater Coastal Plain Brunswick THE COUNTIES AND PHYSIOGRAPHIC PROVINCES OF NORTH CAROLINA Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org This list is dynamic and is revised frequently as new data become available. New species are added to the list, and others are dropped from the list as appropriate. -

Lehman Caves Management Plan
National Park Service U.S. Department of the Interior Great Basin National Park Lehman Caves Management Plan June 2019 ON THE COVER Photograph of visitors on tour of Lehman Caves NPS Photo ON THIS PAGE Photograph of cave shields, Grand Palace, Lehman Caves NPS Photo Shields in the Grand Palace, Lehman Caves. Lehman Caves Management Plan Great Basin National Park Baker, Nevada June 2019 Approved by: James Woolsey, Superintendent Date Executive Summary The Lehman Caves Management Plan (LCMP) guides management for Lehman Caves, located within Great Basin National Park (GRBA). The primary goal of the Lehman Caves Management Plan is to manage the cave in a manner that will preserve and protect cave resources and processes while allowing for respectful recreation and scientific use. More specifically, the intent of this plan is to manage Lehman Caves to maintain its geological, scenic, educational, cultural, biological, hydrological, paleontological, and recreational resources in accordance with applicable laws, regulations, and current guidelines such as the Federal Cave Resource Protection Act and National Park Service Management Policies. Section 1.0 provides an introduction and background to the park and pertinent laws and regulations. Section 2.0 goes into detail of the natural and cultural history of Lehman Caves. This history includes how infrastructure was built up in the cave to allow visitors to enter and tour, as well as visitation numbers from the 1920s to present. Section 3.0 states the management direction and objectives for Lehman Caves. Section 4.0 covers how the Management Plan will meet each of the objectives in Section 3.0. -

Antarctic Bryophyte Research—Current State and Future Directions
Bry. Div. Evo. 043 (1): 221–233 ISSN 2381-9677 (print edition) DIVERSITY & https://www.mapress.com/j/bde BRYOPHYTEEVOLUTION Copyright © 2021 Magnolia Press Article ISSN 2381-9685 (online edition) https://doi.org/10.11646/bde.43.1.16 Antarctic bryophyte research—current state and future directions PAULO E.A.S. CÂMARA1, MicHELine CARVALHO-SILVA1 & MicHAEL STecH2,3 1Departamento de Botânica, Universidade de Brasília, Brazil UnB; �[email protected]; http://orcid.org/0000-0002-3944-996X �[email protected]; https://orcid.org/0000-0002-2389-3804 2Naturalis Biodiversity Center, P.O. Box 9517, 2300 RA Leiden, Netherlands; 3Leiden University, Leiden, Netherlands �[email protected]; https://orcid.org/0000-0001-9804-0120 Abstract Botany is one of the oldest sciences done south of parallel 60 °S, although few professional botanists have dedicated themselves to investigating the Antarctic bryoflora. After the publications of liverwort and moss floras in 2000 and 2008, respectively, new species were described. Currently, the Antarctic bryoflora comprises 28 liverwort and 116 moss species. Furthermore, Antarctic bryology has entered a new phase characterized by the use of molecular tools, in particular DNA sequencing. Although the molecular studies of Antarctic bryophytes have focused exclusively on mosses, molecular data (fingerprinting data and/or DNA sequences) have already been published for 36 % of the Antarctic moss species. In this paper we review the current state of Antarctic bryological research, focusing on molecular studies and conservation, and discuss future questions of Antarctic bryology in the light of global challenges. Keywords: Antarctic flora, conservation, future challenges, molecular phylogenetics, phylogeography Introduction The Antarctic is the most pristine, but also most extreme region on Earth in terms of environmental conditions. -

The Ecology of the in the North York Moors National Park
The ecology of the invasive moss Campylopus introflexus in the North York Moors National Park by Miguel Eduardo Equihua Zamora A thesis presented for the degree of Doctor of Philosophy in the Department of Biology at the University of York November 1991 I hereby declare that the work presented in this thesis is the result of my own investigation and has not been accepted in previous applications for the award of a degree. Exceptions to this declaration are part of the field data used in chapter 4, which was collected and made available to me by Dr. M.B. Usher. The distribution map on Campylopus introflexus was provided by P.T. Harding (Biological Records Centre, ITE, Monks Wood). R.C. Palmer (Soil Survey and Land Research Centre, University of York) made available to me the soil and climatological data of the area, and helped me to obtain the corresponding interpolation values for the sampled sites. Miguel Eduardo Equihua Zamora 1 CONTENTS page Acknowledgements . 4 Abstract................................................. 5 1. Introduction 1.1 The invader: Campylopus introflexus ..................... 7 The invasion of the Northern Hemisphere ............... 7 Taxonomyand identity ............................ 13 Ecology....................................... 16 1.2 The problem ...................................... 19 1.3 Hypothetical mechanisms of interaction ................... 22 2. Aims of the research ......................................28 3. Description of the study area .................................29 4.Ecological preferences of Campylopus introflexus in the North York Moors National Park 4.1 Introduction ....................................... 35 4.2 Methods ......................................... 36 Thefuzzy c-means algorithm ........................ 39 Evaluation of the associations ........................ 43 Desiccation survival of the moss carpets ................ 44 4.3 Results .......................................... 45 Vegetationanalysis ............................... 45 Assessment of moss associations ..................... -

U.S. Strategy for Engagement in Central America: an Overview
Updated February 16, 2021 U.S. Strategy for Engagement in Central America: An Overview Introduction for Engagement in Central America and, with congressional Central America has received considerable attention from support, more than doubled annual foreign aid to the region. U.S. policymakers over the past decade, as it has become a major transit corridor for illicit narcotics and a top source of The Trump Administration repeatedly sought to scale back irregular migration to the United States. In FY2019, U.S. funding for the Central America strategy. It proposed authorities at the southwest border apprehended nearly significant year-on-year assistance cuts for the region in 608,000 unauthorized migrants from El Salvador, each of its annual budget requests and suspended most aid Guatemala, and Honduras (the Northern Triangle of Central for the Northern Triangle in March 2019, two years into the America); 81% of those apprehended were families or strategy’s on-the-ground implementation. Congress chose unaccompanied minors, many of whom were seeking not to adopt many of the proposed cuts, but annual funding asylum (see Figure 1). Although the Coronavirus Disease for the Central America strategy declined from $750 2019 (COVID-19) pandemic disrupted illicit trafficking and million in FY2017 to $505.9 million in FY2021—a nearly irregular migration flows in FY2020, many analysts expect 33% drop over four years (see Figure 2). a resurgence once governments throughout the Western Hemisphere begin lifting border restrictions. Both Congress and the Biden Administration have called for a reexamination of U.S. policy toward Central America Figure 1. U.S. -

Spore Dispersal Vectors
Glime, J. M. 2017. Adaptive Strategies: Spore Dispersal Vectors. Chapt. 4-9. In: Glime, J. M. Bryophyte Ecology. Volume 1. 4-9-1 Physiological Ecology. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 3 June 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 4-9 ADAPTIVE STRATEGIES: SPORE DISPERSAL VECTORS TABLE OF CONTENTS Dispersal Types ............................................................................................................................................ 4-9-2 Wind Dispersal ............................................................................................................................................. 4-9-2 Splachnaceae ......................................................................................................................................... 4-9-4 Liverworts ............................................................................................................................................. 4-9-5 Invasive Species .................................................................................................................................... 4-9-5 Decay Dispersal............................................................................................................................................ 4-9-6 Animal Dispersal .......................................................................................................................................... 4-9-9 Earthworms .......................................................................................................................................... -

Phylogenetic and Morphological Notes on Uleobryum Naganoi Kiguchi Et Ale (Pottiaceae, Musci) 1
HikobiaHikobial4:143-147.2004 14: 143-147.2004 PhylogeneticPhylO窪eneticandmorphOlO=icalmtesⅢIノルCD〃"剛〃昭肌oiKiguchi and morphological notes on Uleobryum naganoi Kiguchi eteraL(POttiaceae,Musci)’ ale (Pottiaceae, Musci) 1 HIROYUKIHIRoYuKISATQHⅡRoMITsuBoTA,ToMIoYAMAGucHIANDHIRoNoRIDEGucH1 SATO, HIROMI TSUBOTA, TOMIO YAMAGUCHI AND HIRONORI DEGUCHI SATO,SATO,H、,TsuBoTA,H、,YAMAGucHI,T、&DEGucHI,H2004Phylogeneticandmor- H., TSUBOTA, H., YAMAGUCHI, T. & DEGUCHI, H. 2004. Phylogenetic and mor phologicalphologicalnotesonU/eo6Mイノ'z〃αgα"ojKiguchietα/、(Pottiaceae,Musci)Hikobia notes on Uleobryum naganoi Kiguchi et al. (Pottiaceae, Musci). Hikobia 14:l4:143-147. 143-147. UleobryumU/eo6/Wm〃αgα"ojKiguchiejα/、,endemictoJapanwithalimitednumberofknown naganoi Kiguchi et aI., endemic to Japan with a limited number of known locations,locations,isnewlyreportedffomShikoku,westernJapanThroughcarefUlexamina- is newly reported from Shikoku, western Japan. Through careful examina tionoffTeshmaterial,rhizoidalmberfbnnationisconfinnedfbrthefirsttime・The , tion of fresh material, rhizoidal tuber formation is confirmed for the first time. The phylogeneticphylogeneticpositionofthiscleistocalpousmossisalsoassessedonthebasisofmaxi- position of this cleistocarpous moss is also assessed on the basis of maxi mummumlikelihoodanalysisof′bcLgenesequences、ThecuITentpositioninthePot- likelihood analysis of rbcL gene sequences. The current position in the Pot tiaceaetiaceaeissUpportedandacloserelationshiptoEpheme'wmslpj""/OS"川ssuggested is supported and a close relationship to -

Globally Widespread Bryophytes, but Rare in Europe
Portugaliae Acta Biol. 20: 11-24. Lisboa, 2002 GLOBALLY WIDESPREAD BRYOPHYTES, BUT RARE IN EUROPE Tomas Hallingbäck Swedish Threatened Species Unit, P.O. Box 7007, SE-75007 Uppsala, Sweden. [email protected] Hallingbäck, T. (2002). Globally widespread bryophytes, but rare in Europe. Portugaliae Acta Biol. 20: 11-24. The need to save not only globally threatened species, but also regionally rare and declining species in Europe is discussed. One rationale of red-listing species regionally is to be preventive and to counteract the local species extinction process. There is also a value in conserving populations at the edge of their geographical range and this is discussed in terms of genetic variation. Another reason is the political willingness of acting locally rather than globally. Among the rare and non-endemic species in Europe, some are rare and threatened both in Europe and elsewhere, others are more common outside Europe and a third group is locally common within Europe but rare in the major part. How much conservation effort should be put on these three European non-endemic species groups is briefly discussed, as well as why bryophytes are threatened. A discussion is given, for example, of how a smaller total distribution range, decreasing density of localities, smaller sites, less substrate and lower habitat quality affect the survival of sensitive species. This is also compared with species that have either high or low dispersal capacity or different longevity of either vegetative parts or spores. Examples from Sweden are given. Key words: Bryophytes, rarity, Europe, dispersal capacity, Sweden. Hallingbäck, T. (2002). -

New National and Regional Bryophyte Records, 63
Journal of Bryology ISSN: 0373-6687 (Print) 1743-2820 (Online) Journal homepage: https://www.tandfonline.com/loi/yjbr20 New national and regional bryophyte records, 63 L. T. Ellis, O. M. Afonina, I. V. Czernyadjeva, L. A. Konoreva, A. D. Potemkin, V. M. Kotkova, M. Alataş, H. H. Blom, M. Boiko, R. A. Cabral, S. Jimenez, D. Dagnino, C. Turcato, L. Minuto, P. Erzberger, T. Ezer, O. V. Galanina, N. Hodgetts, M. S. Ignatov, A. Ignatova, S. G. Kazanovsky, T. Kiebacher, H. Köckinger, E. O. Korolkova, J. Larraín, A. I. Maksimov, D. Maity, A. Martins, M. Sim-Sim, F. Monteiro, L. Catarino, R. Medina, M. Nobis, A. Nowak, R. Ochyra, I. Parnikoza, V. Ivanets, V. Plášek, M. Philippe, P. Saha, Md. N. Aziz, A. V. Shkurko, S. Ştefănuţ, G. M. Suárez, A. Uygur, K. Erkul, M. Wierzgoń & A. Graulich To cite this article: L. T. Ellis, O. M. Afonina, I. V. Czernyadjeva, L. A. Konoreva, A. D. Potemkin, V. M. Kotkova, M. Alataş, H. H. Blom, M. Boiko, R. A. Cabral, S. Jimenez, D. Dagnino, C. Turcato, L. Minuto, P. Erzberger, T. Ezer, O. V. Galanina, N. Hodgetts, M. S. Ignatov, A. Ignatova, S. G. Kazanovsky, T. Kiebacher, H. Köckinger, E. O. Korolkova, J. Larraín, A. I. Maksimov, D. Maity, A. Martins, M. Sim-Sim, F. Monteiro, L. Catarino, R. Medina, M. Nobis, A. Nowak, R. Ochyra, I. Parnikoza, V. Ivanets, V. Plášek, M. Philippe, P. Saha, Md. N. Aziz, A. V. Shkurko, S. Ştefănuţ, G. M. Suárez, A. Uygur, K. Erkul, M. Wierzgoń & A. Graulich (2020): New national and regional bryophyte records, 63, Journal of Bryology, DOI: 10.1080/03736687.2020.1750930 To link to this article: https://doi.org/10.1080/03736687.2020.1750930 Published online: 18 May 2020. -

A Taxonomic Revision of Dicranodontium(Musci)
Ann. Bot. Fennici 34: 179–204 ISSN 0003-3847 Helsinki 19 September 1997 © Finnish Zoological and Botanical Publishing Board 1997 A taxonomic revision of Dicranodontium (Musci) Jan-Peter Frahm Frahm, J.-P., Botanisches Institut der Universität, Meckenheimer Allee 170, D-51115 Bonn, Germany Received 23 December 1996, accepted 26 February 1997 The 39 species of Dicranodontium which are currently included in the genus are criti- cally revised and reduced to 7. Dicranodontium fleischerianum Schultze-Motel could not be separated from D. uncinatum (Harv.) Jaeg. and is regarded as synonymous with the latter. Dicranodontium dimorphum Mitt. has proven to be synonymous with D. didictyon (Mitt.) Jaeg., D. meridionale Bartr. with D. pulchroalare Broth., D. cey- lonense Fleisch., D. subasperum Williams and D. perviride Dix. & P. Varde are syn- onymous with D. denudatum (Brid.) Britt., D. subintegrifolium Broth., D. attenuatum (Mitt.) Wils., D. caespitosum (Mitt.) Par. and D. decipiens (Mitt.) Mitt. ex Broth. are synonymous with D. didymodon (Griff.) Par., D. blindioides (Besch.) Broth., D. sparsum Dix. are synonymous with D. uncinatum (Harv.) Jaeg. Dicranodontium sinense (C. Müll.) Par. is identical with Campylopus japonicus Broth. and recognized as Campylopus sinensis (C. Müll.) J.-P. Frahm comb. nov. Dicranodontium falcatum Broth. (described as endemic from Hawaii) is synonymous with D. porodictyon Card. & Thér. from China, D. tanganyikae J. Tayl. & P. Varde is identical with Campylopus flexuosus (Hedw.) Brid. var. incacorralis (Herz.) J.-P. Frahm, D. humilis P. Varde is a species of Blindia, D. interruptum P. Varde is identical with Bryohumbertia filifolia (Hornsch.) J.-P. Frahm, D. horricuspis Card. and D. capillifolium (Dix.) Takaki are synonymous with D.