Fundamentals of Enzyme Kinetics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Author Version (PDF)
Chemical Society Reviews Understanding Catalysis Journal: Chemical Society Reviews Manuscript ID: CS-TRV-06-2014-000210.R2 Article Type: Tutorial Review Date Submitted by the Author: 20-Jun-2014 Complete List of Authors: Roduner, Emil; Universitat Stuttgart, Institut fur Physikalische Chemie Page 1 of 33 Chemical Society Reviews Tutorial Review Understanding Catalysis Emil Roduner Institute of Physical Chemistry, University of Stuttgart, D-70569 Stuttgart, Germany and Chemistry Department, University of Pretoria, Pretoria 0002, Republic of South Africa Abstract The large majority of chemical compounds underwent at least one catalytic step during synthesis. While it is common knowledge that catalysts enhance reaction rates by lowering the activation energy it is often obscure how catalysts achieve this. This tutorial review explains some fundamental principles of catalysis and how the mechanisms are studied. The dissociation of formic acid into H 2 and CO 2, serves to demonstrate how a water molecule can open a new reaction path at lower energy, how immersion in liquid water can influence the charge distribution and energetics, and how catalysis at metal surfaces differs from that in the gas phase. The reversibility of catalytic reactions, the influence of an adsorption pre- equilibrium and the compensating effects of adsorption entropy and enthalpy on the Arrhenius parameters are discussed. It is shown that flexibility around the catalytic centre and residual substrate dynamics on the surface affect these parameters. Sabatier’s principle of optimum substrate adsorption, shape selectivity in the pores of molecular sieves and the polarisation effect at the metal-support interface are explained. Finally, it is shown that the application of a bias voltage in electrochemistry offers an additional parameter to promote or inhibit a reaction. -

Eyring Equation
Search Buy/Sell Used Reactors Glass microreactors Hydrogenation Reactor Buy Or Sell Used Reactors Here. Save Time Microreactors made of glass and lab High performance reactor technology Safe And Money Through IPPE! systems for chemical synthesis scale-up. Worldwide supply www.IPPE.com www.mikroglas.com www.biazzi.com Reactors & Calorimeters Induction Heating Reacting Flow Simulation Steam Calculator For Process R&D Laboratories Check Out Induction Heating Software & Mechanisms for Excel steam table add-in for water Automated & Manual Solutions From A Trusted Source. Chemical, Combustion & Materials and steam properties www.helgroup.com myewoss.biz Processes www.chemgoodies.com www.reactiondesign.com Eyring Equation Peter Keusch, University of Regensburg German version "If the Lord Almighty had consulted me before embarking upon the Creation, I should have recommended something simpler." Alphonso X, the Wise of Spain (1223-1284) "Everything should be made as simple as possible, but not simpler." Albert Einstein Both the Arrhenius and the Eyring equation describe the temperature dependence of reaction rate. Strictly speaking, the Arrhenius equation can be applied only to the kinetics of gas reactions. The Eyring equation is also used in the study of solution reactions and mixed phase reactions - all places where the simple collision model is not very helpful. The Arrhenius equation is founded on the empirical observation that rates of reactions increase with temperature. The Eyring equation is a theoretical construct, based on transition state model. The bimolecular reaction is considered by 'transition state theory'. According to the transition state model, the reactants are getting over into an unsteady intermediate state on the reaction pathway. -

The 'Yard Stick' to Interpret the Entropy of Activation in Chemical Kinetics
World Journal of Chemical Education, 2018, Vol. 6, No. 1, 78-81 Available online at http://pubs.sciepub.com/wjce/6/1/12 ©Science and Education Publishing DOI:10.12691/wjce-6-1-12 The ‘Yard Stick’ to Interpret the Entropy of Activation in Chemical Kinetics: A Physical-Organic Chemistry Exercise R. Sanjeev1, D. A. Padmavathi2, V. Jagannadham2,* 1Department of Chemistry, Geethanjali College of Engineering and Technology, Cheeryal-501301, Telangana, India 2Department of Chemistry, Osmania University, Hyderabad-500007, India *Corresponding author: [email protected] Abstract No physical or physical-organic chemistry laboratory goes without a single instrument. To measure conductance we use conductometer, pH meter for measuring pH, colorimeter for absorbance, viscometer for viscosity, potentiometer for emf, polarimeter for angle of rotation, and several other instruments for different physical properties. But when it comes to the turn of thermodynamic or activation parameters, we don’t have any meters. The only way to evaluate all the thermodynamic or activation parameters is the use of some empirical equations available in many physical chemistry text books. Most often it is very easy to interpret the enthalpy change and free energy change in thermodynamics and the corresponding activation parameters in chemical kinetics. When it comes to interpretation of change of entropy or change of entropy of activation, more often it frightens than enlightens a new teacher while teaching and the students while learning. The classical thermodynamic entropy change is well explained by Atkins [1] in terms of a sneeze in a busy street generates less additional disorder than the same sneeze in a quiet library (Figure 1) [2]. -

CHM 8304 Physical Organic Chemistry
CHM 8304 CHM 8304 Physical Organic Chemistry Kinetic analyses Scientific method • proposal of a hypothesis • conduct experiments to test this hypothesis – confirmation – refutation • the trait of refutability is what distinguishes a good scientific hypothesis from a pseudo-hypothesis (see Karl Popper) 2 Kinetic analyses 1 CHM 8304 “Proof” of a mechanism • a mechanism can never really be ‘proven’ – inter alia, it is not directly observable! • one can propose a hypothetical mechanism • one can conduct experiments designed to refute certain hypotheses • one can retain mechanisms that are not refuted • a mechanism can thus become “generally accepted” 3 Mechanistic studies • allow a better comprehension of a reaction, its scope and its utility • require kinetic experiments – rate of disappearance of reactants – rate of appearance of products • kinetic studies are therefore one of the most important disciplines in experimental chemistry 4 Kinetic analyses 2 CHM 8304 Outline: Energy surfaces • based on section 7.1 of A&D – energy surfaces – energy profiles – the nature of a transition state – rates and rate constants – reaction order and rate laws 5 Energy profiles and surfaces • tools for the visualisation of the change of energy as a function of chemical transformations – profiles: • two dimensions • often, energy as a function of ‘reaction coordinate’ – surfaces: • three dimensions • energy as a function of two reaction coordinates 6 Kinetic analyses 3 CHM 8304 Example: SN2 energy profile • one step passing by a single transition state δ- -

Introduction to the Transition State Theory 29
DOI: 10.5772/intechopen.78705 ProvisionalChapter chapter 2 Introduction toto thethe TransitionTransition StateState TheoryTheory Petr PtáPtáček,ček, FrantiFrantišekšek ŠŠoukaloukal andand TomáTomášš OpravilOpravil Additional information isis availableavailable atat thethe endend ofof thethe chapterchapter http://dx.doi.org/10.5772/intechopen.78705 Abstract The transition state theory (TST), which is also known as theory of absolute reaction rates (ART) and the theory of activated state (complex), is essentially a refined version of crude collision theory, which treats the reacting molecules as the rigid spheres without any internal degree of freedom. The theory explains the rate of chemical reaction assuming a special type of chemical equilibrium (quasi-equilibrium) between the reactants and acti- vated state (transition state complex). This special molecule decomposes to form the products of reaction. The rate of this reaction is then equal to the rate of decomposition of activated complex. This chapter also explains the limitation of TST theory and deals with the kinetics isotope effect. Keywords: transition state theory, theory of absolute reaction rates, activated complex, equilibrium constant, reaction rate, kinetics, activation energy, frequency factor, partition function, thermodynamics of activated state, kinetics isotope effect 1. Introduction “Two major findings have, however, stood the test of time. First, there was the unmistakable evidence that molecular collisions play the all-important role in communicating the activation energy to the molecules which are to be transformed and, second, there was the recognition of the activation energy (derivable for the Arrhenius Law of the temperature dependence) as a dominant, though by no means the sole, factor determining reactivity in general.”—C.N. -

Organic Chemistry Topic : General Treatment of Reaction Mechanism II (Reaction Kinetics)
Teacher: DR. SUBHANKAR SARDAR Class : Semester-2 Paper: C4T: Organic Chemistry Topic : General Treatment of Reaction Mechanism II (Reaction kinetics) Comments: Read the notes thoroughly. This part is very important for final examination. References: Wikipedia General Tratment of Reaction Mechanism II Reaction Kinetics: Part-1 Chemical kinetics is the branch of physical chemistry which deals with a study of the speed of chemical reactions. Such studies also enable us to understand the mechanism by which the reaction occurs. Thus, in chemical kinetics we can also determine the rate of chemical reaction. From the kinetic stand point the reactions are classified into two groups: a) homogeneous reactions which occur entirely in one phase b) heterogeneous reactions where the transformation takes place on the surface of a catalyst or the walls of a container. Rate of reaction The rate of reaction i.e. the velocity of a reaction is the amount of a chemical change occurring per unit time. The representation of rate of reaction in terms of concentration of the reactants is known as rate law . It is also called as rate equation or rate expression.The rate is generally expressed as the decrease in concentration of a reactant or as the increase in concentration of the product. If C the concentration of a reactant at any time t is, the rate is expressed by -dC/dt or if the concentration of a product be x at any time t, the rate would be dx/dt . The time is usually expressed in seconds. The rate will have units of concentration divided by time. -
Activated Complex Theory
CHAPTER 3 Chemical Dynamics – I 143 Activated Complex Theory In 1935, an American chemist Henry Eyring; alongside two British chemists, Meredith Gwynne Evans and Michael Polanyi; proposed a new theory to rationalize the rate of different chemical reactions which was based upon the formation of an activated intermediate complex. This theory is also known as the "transition state theory", "theory of absolute reaction rates”, and "absolute-rate theory". The activated complex theory states that the rates of various elementary chemical reactions can be explained by assuming a special type of chemical equilibria (quasi-equilibrium) between reactants and activated complexes. Before the development of activated complex theory, the Arrhenius rate law was popularly used to determine energies for the potential barrier. However, the Arrhenius equation was based on empirical observations rather than mechanistic investigations as if one or more intermediates are involved in the conversion or not. For that reason, more development was essential to know the two factors present in the Arrhenius equation, the activation energy (Ea) and the pre-exponential factor (A). The Eyring equation from transition state theory successfully addresses these two issues and therefore contributed significantly to the conceptual understanding of reaction kinetics. Figure 11. The variation of free energy as the reaction proceeds. To explore the concept mathematically, consider a reaction between reactant A and B forming a product P via the activated complex X* as: ∗ 퐾 푘 (178) 퐴 + 퐵 ⇌ 푋∗ → 푃 The equilibrium constant for reactants to activated complex conversion is [푋∗] (179) 퐾∗ = [퐴][퐵] Copyright © Mandeep Dalal 144 A Textbook of Physical Chemistry – Volume I Henry Eyring showed that the rate constant for a chemical reaction with any order or molecularity can be given by the following relation. -

The Development of the Arrhenius Equation
The Development of the Arrhenius Equation Keith J. Laidler Onawa-Carleton Institute for Research and Graduate Studies in Chemistry, University of Onawa, Onawa, Ontario. Canada KIN 684 Todav the influence of temperature on the rate of chemical k = AFn (1 +GO) (4) reactions is almost always interpreted in terms of what is now known as the Arrhenius equation. According to this, a rate In terms of the absolute temperature T, the equation can he constant h is the product of a pre- exponentid ("frequency") written as factor A and an exponential term k = A'FT(l + G'T) (5) k = A~-EIRT (1) In 1862 Berthelot (9) presented the equation where R is the gas constant and E is the activation energy. The Ink=A'+DT or k = AeDT (6) apparent activation energy E.,, is now defined (I) in terms of this equation as A ronsi(lrrable niimlwr of workers bupportrd thls equation, which was taken seriously at least until 19011 (101. In 1881 Warder (11, 12) prop,wd thr reli~tlonshlp (a k)(b T) = e Often A and E can he treated as temperature independent. + - (7) For the analvsis of more precise rate-temperature data, par- where a, b, and c are constants, and later Mellor (4) pointed ticularly those covering a wide temperature range, it is usual out that if one multiplies out this formula, expands the factor to allow A to he proportional to T raised to a power m, so that in T and accepts only the first term, the result is the equation hecomes k = a' + b'T2 (8) k = A'T~~~-E/RT (3) Equation (7) received some support from results obtained by where now A' is temperature independent. -

In Collision Theory, the Detailed Nature of the Interactions Between Reactants Was Not Considered
Module 7 : Theories of Reaction Rates Lecture 33 : Transition State Theory Objectives After studying this Lecture you will be able to do the following. Distinguish between collision theory and transition state theory. Obtain the rate constant in terms of an equilibrium constant of the transition state. Express the transition state equilibrium constant in terms of the partition functions of the transition state and the reactants. Associate the rate constant for the reaction {transition state products} with the vibrational frequency of the transition state. Express the partition function of the transition state as a product of the partition functions for translational and other modes. Define free energy, entropy and enthalpy of activation. Rationalise the isotope effect on reaction rates. 33.1 Introduction In collision theory, the detailed nature of the interactions between reactants was not considered. The detailed interactions are best represented through the potential energy surface which will be considered in the next lecture. From the Arrhenius equation, only those collisions with the minimum required energy can lead to the products. Consider one such path which is represented in Fig 33.1. Figure 33.1 Potential energy of reaction as a function of the reaction coordinate. The ordinate is the potential energy (PE) and the absissa is the reaction coordinate. At a specific state (configuration of the reactants), the potential energy is maximum, the slope is zero and the PE falls to lower values in both the forward and the reverse direction. All the structures in the vicinity of this transition state may be considered as the "activated complex", which is very reactive. -

The Mechanism of Adsorption of Rh(III) Bromide Complex Ions on Activated Carbon
molecules Article The Mechanism of Adsorption of Rh(III) Bromide Complex Ions on Activated Carbon Marek Wojnicki 1,* , Andrzej Krawontka 1, Konrad Wojtaszek 1, Katarzyna Skibi ´nska 1 , Edit Csapó 2,3 , Zbigniew P˛edzich 4 , Agnieszka Podborska 5 and Przemysław Kwolek 6 1 Faculty of Non-Ferrous Metals, AGH University of Science and Technology, Mickiewicza Ave. 30, 30-059 Krakow, Poland; [email protected] (A.K.); [email protected] (K.W.); [email protected] (K.S.) 2 MTA-SZTE Biomimetic Systems Research Group, University of Szeged, H-6720 Dóm tér 8, 6720 Szeged, Hungary; [email protected] 3 Interdisciplinary Excellence Centre, Department of Physical Chemistry and Materials Science, University of Szeged, Rerrich B. tér 1, H-6720 Szeged, Hungary 4 Faculty of Materials Science and Ceramics, AGH University of Science and Technology, al. A. Mickiewicza 30, 30-059 Krakow, Poland; [email protected] 5 Academic Centre for Materials and Nanotechnology, AGH University of Science and Technology, al. A. Mickiewicza 30, 30-059 Krakow, Poland; [email protected] 6 Department of Materials Science, Faculty of Mechanical Engineering and Aeronautics, Rzeszow University of Technology, 35-959 Rzeszow, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-126-174-126; Fax: +48-126-332-316 Abstract: In the paper, the mechanism of the process of the Rh(III) ions adsorption on activated carbon ORGANOSORB 10—AA was investigated. It was shown, that the process is reversible, Citation: Wojnicki, M.; Krawontka, i.e., stripping of Rh(III) ions from activated carbon to the solution is also possible. -
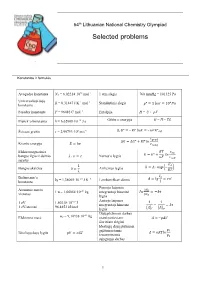
Selected Problems
54th Lithuanian National Chemistry Olympiad Selected problems . Konstantos ir formulės 23 –1 Avogadro konstanta NA = 6,02214·10 mol 1 atm slėgis 760 mmHg = 101325 Pa Universalioji dujų R = 8,31447 J K–1 mol–1 Standartinis slėgis 푝° = 1 푏푎푟 = 105푃푎 konstanta Faraday konstanta F = 96485 C mol–1 Entalpija H = U + pV Planck‘o konstanta h = 6,62608·10–34 J s Gibbs‘o energija G = H – TS Šviesos greitis c = 2,99793·108 m s–1 ∆푟퐺° = – 푅푇 푙푛퐾 = – 푛퐹퐸°푐푒푙 푐푝푟표푑 ∆퐺 = ∆퐺° + 푅푇 푙푛 Kvanto energija 퐸 = ℎ푣 푐푟푒푎푔 Elektromagnetinės 푅푇 푐표푥 bangos ilgio ir dažnio 휆 · 푣 = 푐 Nernst‘o lygtis 퐸 = 퐸° + 푙푛 푛퐹 푐푟푒푑 sąryšis 1 퐸퐴 Bangos skaičius ῦ = Arrhenius lygtis 푘 = 퐴 · exp (– ) 휆 푅푇 Boltzmann‘o 퐼표 k = 1,38065·10–23 J K–1 Lambert-Beer dėsnis 퐴 = 푙푔 = 휀푐푙 konstanta B 퐼 Pirmojo laipsnio Atominės masės [퐴]푡 1 u = 1,66054·10–27 kg integruotoji kinetinė 푙푛 = −푘푡 vienetas [퐴] lygtis 표 Antrojo laipsnio 1 eV 1,60218·10–19 J 1 1 integruotoji kinetinė − = 푘푡 1 eV/atomui 96,4853 kJ/mol lygtis [퐴]푡 [퐴]표 –31 Dujų plėtimosi darbas Elektrono masė me = 9,10938∙10 kg esant pastoviam 퐴 = – 푝∆푉 išoriniam slėgiui Idealiųjų dujų plėtimosi grįžtamosiomis 푝2 Idealiųjų dujų lygtis 푝푉 = 푛푅푇 퐴 = 푛푅푇푙푛 izoterminėmis 푝1 sąlygomis darbas 1 Problem 1. LINALOOL Linalool – a common fragrance compound, found in cosmetics: soaps, parfume, shampoos, etc. It is estimated that around 60- 80% of products contain this organic compound and the commercial synthesis of linalool is important in order to fullfil the demand since natural sources are scarce.