7. References
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogeny of Endopterygote Insects, the Most Successful Lineage of Living Organisms*
REVIEW Eur. J. Entomol. 96: 237-253, 1999 ISSN 1210-5759 Phylogeny of endopterygote insects, the most successful lineage of living organisms* N iels P. KRISTENSEN Zoological Museum, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen 0, Denmark; e-mail: [email protected] Key words. Insecta, Endopterygota, Holometabola, phylogeny, diversification modes, Megaloptera, Raphidioptera, Neuroptera, Coleóptera, Strepsiptera, Díptera, Mecoptera, Siphonaptera, Trichoptera, Lepidoptera, Hymenoptera Abstract. The monophyly of the Endopterygota is supported primarily by the specialized larva without external wing buds and with degradable eyes, as well as by the quiescence of the last immature (pupal) stage; a specialized morphology of the latter is not an en dopterygote groundplan trait. There is weak support for the basal endopterygote splitting event being between a Neuropterida + Co leóptera clade and a Mecopterida + Hymenoptera clade; a fully sclerotized sitophore plate in the adult is a newly recognized possible groundplan autapomorphy of the latter. The molecular evidence for a Strepsiptera + Díptera clade is differently interpreted by advo cates of parsimony and maximum likelihood analyses of sequence data, and the morphological evidence for the monophyly of this clade is ambiguous. The basal diversification patterns within the principal endopterygote clades (“orders”) are succinctly reviewed. The truly species-rich clades are almost consistently quite subordinate. The identification of “key innovations” promoting evolution -

Ultrastructural Changes in Female Reproductive Organ of Chrotogonus Trachypterus Blanchard Induced by Deltamethrin
IOSR Journal of Agriculture and Veterinary Science (IOSR-JAVS) e-ISSN: 2319-2380, p-ISSN: 2319-2372. Volume 7, Issue 5 Ver. II (May. 2014), PP 01-06 www.iosrjournals.org Ultrastructural changes in female reproductive organ of Chrotogonus trachypterus Blanchard induced by deltamethrin Shashi Meena1 & N. P. Singh2 Centre for Advanced Studies in Zoology, University of Rajasthan, Jaipur-302055, Rajasthan, India Abstract: Acridid grasshopper, Chrotogonus trachypterus Blanchard is known as surface grasshopper and is a most common polyphagous pest occurring throughout year causing significant damage to seedlings of crops and vegetables. Ultrastructural changes in the ovarian follicles of C. trachypterus Blanchard induced by deltamethrin one day after treatment were observed. Orthopteran insects have panoistic ovarioles and each of the paired ovary consists of tubular ovarioles along which are placed the oocytes in linear sequence that reflexes their progressive development. Each ovariole is divided into a terminal filament, germarium and a vitellarium. In the present study electron micrographs of ovarian follicle cells of females treated with deltamethrin showed prominent histopathological changes leading to vacuolization of cytoplasm, degeneration of the cell components of follicular epithelium and most obvious signs were observed of yolk damage and mitochondrial disintegration, when examined by transmission electron microscopy (TEM). The present study indicates a profound effect on reproduction of the pest by deltamethrin, a synthetic pyrethroid and suggests alternative of more hazardous synthetic organic insecticides. Key Words: Chrotogonus trachypterus Blanchard, ovarian follicle, synthetic pyrethroid, deltamethrin histopathological changes, transmission electron microscopy (TEM) I. Introduction The surface grasshopper, Chrotogonus trachypterus Blanchard (Orthoptera: Acrididae) has been recognized as a threat to agricultural in semi arid zone of Rajasthan, India. -

The Gloucester Mecopteran
The Gloucester Mecopteran Vol 1, No 4: April 2015 Editorial: Journals and newsletters generally try to provide their readers with up to date content, but on this occasion The Gloucester Mecopteran makes no apology for stepping back through a few hundred million years. Any sensitive mecopterist must surely regret the sticky fate of the ancient scorpionfly relative in our front page image. However, this individual misfortune has preserved for us a glimpse into the long, significant history of a noble hexapod clan. * * * The editor’s thanks go to The Gloucestershire Naturalists’ Society for reproducing the first two issues of The Gloucester Mecopteran in The Gloucestershire Naturalist No. 26 (2014), and also to Worcestershire Biological Records Centre and Worcestershire Recorders for their continuing interest and support. Hylobittacus fossilis? in Baltic Eocene amber A brief history of scorpionflies © Marius Veta (Lithuania) Quite recently, but before a climate change triggered placental mammals were also alive. Taxonomists the Ice Ages that shaped our modern world, a warm place Mecoptera close to the roots of a group of forest stretched across Northern Europe. Resin ooz- insect orders including caddisflies, butterflies and ing from the trees trapped many insects, creating a moths, fleas and true flies that first appeared in the legacy of fossils in amber. The stunning portrait above Mesozoic. It was probably creatures very like our is a reminder that delightful species of Mecoptera familiar scorpionflies that gave rise to this diverse were scavenging for food through a succession of range of descendants. geological ages, all very different from our own times. The last Mesozoic period, the Cretaceous, is most The first known Mecoptera lived much earlier, in the famous for its catastrophic end, marked by the Permian, the last period of the Palaeozoic era which extinction of the dinosaurs, but it also saw the first ended with a massive extinction event. -

Fleas Are Parasitic Scorpionflies
Palaeoentomology 003 (6): 641–653 ISSN 2624-2826 (print edition) https://www.mapress.com/j/pe/ PALAEOENTOMOLOGY PE Copyright © 2020 Magnolia Press Article ISSN 2624-2834 (online edition) https://doi.org/10.11646/palaeoentomology.3.6.16 http://zoobank.org/urn:lsid:zoobank.org:pub:9B7B23CF-5A1E-44EB-A1D4-59DDBF321938 Fleas are parasitic scorpionflies ERIK TIHELKA1, MATTIA GIACOMELLI1, 2, DI-YING HUANG3, DAVIDE PISANI1, 2, PHILIP C. J. DONOGHUE1 & CHEN-YANG CAI3, 1, * 1School of Earth Sciences University of Bristol, Life Sciences Building, Tyndall Avenue, Bristol, BS8 1TQ, UK 2School of Life Sciences University of Bristol, Life Sciences Building, Tyndall Avenue, Bristol, BS8 1TQ, UK 3State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, and Centre for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, Nanjing 210008, China [email protected]; https://orcid.org/0000-0002-5048-5355 [email protected]; https://orcid.org/0000-0002-0554-3704 [email protected]; https://orcid.org/0000-0002-5637-4867 [email protected]; https://orcid.org/0000-0003-0949-6682 [email protected]; https://orcid.org/0000-0003-3116-7463 [email protected]; https://orcid.org/0000-0002-9283-8323 *Corresponding author Abstract bizarre bodyplans and modes of life among insects (Lewis, 1998). Flea monophyly is strongly supported by siphonate Fleas (Siphonaptera) are medically important blood-feeding mouthparts formed from the laciniae and labrum, strongly insects responsible for spreading pathogens such as plague, murine typhus, and myxomatosis. The peculiar morphology reduced eyes, laterally compressed wingless body, of fleas resulting from their specialised ectoparasitic and hind legs adapted for jumping (Beutel et al., 2013; lifestyle has meant that the phylogenetic position of this Medvedev, 2017). -

The New York Forest Owner a Publication of the New York Forest Owners Association for People Caring About New York’S Trees and Forests May/June 2014
The New York Forest Owner A PUBLICATION OF THE NEW YORK FOREST OWNERS ASSOCIATION For people caring about New York’s trees and forests May/June 2014 Member Profile: Gary Goff Volume 52 Number 3 www.nyfoa.org THE NEW YORK In This Issue . FOREST OWNERS FROM THE PRESIDENT JIM MINOR ..................................................................................................... 3 ASSOCIATION UPDATE ON RNYW 2014 Officers & Directors JERRY MICHAEL ............................................................................................ 5 Jim Minor, President 22 Bryn Mawr Rd ASK A PROFESSIONAL Rochester, NY 14624; (585) 247-7069 PETER SMALLIDGE ......................................................................................... 6 [email protected] Ron Pedersen, Vice President NEW YORK STATE TREE FARM NEWS 22 Vandenburg Lane ERIN O’NEILL ............................................................................................. 8 Latham, NY 12110; (518) 785-6061 [email protected] KIDS CORNER DEREK J. CONANT ....................................................................................... 9 Sarah Stackhouse, Secretary/Treasurer 3010 Esperanza Rd Bluff Point, NY 14478; (315) 536-9482 WILD THINGS IN YOUR WOODLANDS [email protected] KRISTI SULLIVAN ........................................................................................... 10 Renee Bouplon, Cambridge, (518) 692-7285. 2016 EQIP COST-SHARING AVAILABLE FOR FOREST STEWARDSHIP Bob Glidden, Niagara Frontier, (716) 795-3305 JERRY MICHAEL ........................................................................................... -

Far Eastern Entomologist Number 359: 12-15 ISSN 1026-051X May 2018
Far Eastern Entomologist Number 359: 12-15 ISSN 1026-051X May 2018 https://doi.org/10.25221/fee.359.3 http/urn:lsid:zoobank.org:pub:09AF614A-40FF-4DB6-94BC-7D974FE8CFF2 NEW DATA ON DISTRIBUTION OF WINTER INSECTS (MECOPTERA: BOREIDAE; DIPTERA: LIMONIIDAE) IN WESTERN SIBERIA V. A. Stolbov*1), D. E. Galich2), D. S. Nizovtsev1) 1) Tyumen State University, Tyumen 625003, Russia. *Corresponding author. E-mail: [email protected] 2) TyumenNIIproekt, Tyumen 625046, Russia. E-mail: [email protected] Summary. New data on the distribution of the winter insects in Western Siberia are given. Snow scorpionfly Boreus westwoodi Hagen, 1866 is recorded from Western Siberia and Asia. Chionea araneoides Dalman, 1816 and Chionea crassipes crassipes Boheman, 1846 are new for the fauna of Tyumen oblast. Key words: Mecoptera, Boreus, Diptera, Chionea, fauna, new record, Tyumen oblast, Russia. В. А. Столбов, Д. Е. Галич, Д. С. Низовцев. Новые данные по распро- странению зимних насекомых (Mecoptera: Boreidae; Diptera: Limoniidae) в Западной Сибири // Дальневосточный энтомолог. 2018. N 359. С. 12-15. Резюме. Приведены новые данные по распространению представителей зимней энтомофауны в Западной Сибири. Ледничник Boreus westwoodi Hagen, 1866 впервые приводится для Западной Сибири и Азии. Chionea araneoides Dalman, 1816 и Chionea crassipes crassipes Boheman, 1846 впервые указываются для Тюменской области. INTRODUCTION Among the huge variety of insects, only a small assembly group of insects is capable of active lifestyles during the winter period. The most typical representatives of the obligatory winter entomofauna are snow scorpionflies (Mecoptera: Boreidae) and snow flies (Diptera: Limoniidae, Chionea spp.). Imagoes of these species are mainly active in winter period and frequently found on snow (Savchenko, 1969; Pavlov, 2006; Oosterbroek & Reusch, 2008). -

A Review of Recent Phylogenetic Contributions
ACTA ENTOMOLOGICA MUSEI NATIONALIS PRAGAE Published 8.xii.2008 Volume 48(2), pp. 217-232 ISSN 0374-1036 Four chapters about the monophyly of insect ‘orders’: A review of recent phylogenetic contributions Jan ZRZAVÝ Department of Zoology, Faculty of Science, University of South Bohemia, and Biology Centre, Czech Academy of Science, Branišovská 31, CZ-370 05 České Budějovice, Czech Republic; e-mail: [email protected] Abstract. Recent phylogenetic analyses, both morphological and molecular, strongly support the monophyly of most insect ‘orders’. On the contrary, the Blattaria, Psocoptera, and Mecoptera are defi nitely paraphyletic (with respect of the Isoptera, Phthiraptera, and Siphonaptera, respectively), and the Phthiraptera are possibly diphyletic. Small relictual subclades that are closely related to the Isoptera, Phthiraptera, and Siphonaptera were identifi ed (Cryptocercidae, Lipo- scelididae, and Boreidae, respectively), which provides an enormous amount of evidence about the origin and early evolution of the highly apomorphic eusocial or parasitic ex-groups. Position of the enigmatic ‘zygentoman’ Tricholepidion Wygodzinsky, 1961, remains uncertain. Possible non-monophyly of the Megalo- ptera (with respect of the Raphidioptera) and the Phasmatodea (with respect of the Embioptera) are shortly discussed. Key words. Insecta, Zygentoma, Tricholepidion, Blattaria, Isoptera, Cryptocercus, Psocoptera, Phthiraptera, Liposcelididae, Mecoptera, Siphonaptera, Boreidae, Nannochoristidae, Timema, phylogeny, monophyly Introduction The goal of modern systematics is twofold: to provide a biological ‘lingua franca’ that facilitates an exchange of information among researchers, and to provide a hierarchical system that is meaningful in the context of our understanding of phylogenetic history. However, both goals are often in confl ict. Phylogenetics is about a nested hierarchy of clades, without any privileged ‘rank’ (like ‘order’ or ‘family’). -

NATURAL RESOURCES COMMISSION Information Bulletin #2
Indiana Register NATURAL RESOURCES COMMISSION Information Bulletin #2 (Eighth Amendment) December 1, 2017 SUBJECT: Roster of Indiana Animals, Insects, and Plants that are Extirpated, Endangered, Threatened, or Rare (also described as Special Concern). I. HISTORY The initial roster was published February 1, 1992 (15 IR 848), republished April 1, 1991 (15 IR 1312); and subsequently amended to include additional species and published on February 1, 2005 (28 IR 1581). Since 2005, revisions have been made to several of the endangered species lists. The term "special concern" replaced the references to "rare" as it relates to wild animals and is expanded to include species in a legal status transition. Federal funding is available for species that are endangered or of special concern. In the fourth amendment, posted at 20070815-IR-312070469NRA on August 15, 2007, "rare", "threatened", and "extirpated" classifications for insect species were retained. Classification definitions were added for vascular plant species. In this document, species are reclassified and scientific names were modified. In the fifth amendment, posted at 20120125-IR-312120047NRA on January 25, 2012, the listing for endangered reptiles and amphibians was modified. Notable was removal of the American bald eagle from the endangered species list. Some species removed from the endangered list were redesignated as "special concern". Amendments were made to use scientific names that are consistent with those for species of animals listed in 312 IAC 9. In the sixth amendment, posted at 20140129-IR-312140023NRA on January 29, 2014, the listing for endangered birds, mollusks, insects, and vascular plants was modified. Notable was the removal of the peregrine falcon from the endangered species list. -

List of Native and Naturalized Fauna of Virginia
Virginia Department of Wildlife Resources List of Native and Naturalized Fauna of Virginia August, 2020 (* denotes naturalized species; ** denotes species native to some areas of Virginia and naturalized in other areas of Virginia) Common Name Scientific Name FISHES: Freshwater Fishes: Alabama Bass * Micropterus henshalli * Alewife Alosa pseudoharengus American Brook Lamprey Lampetra appendix American Eel Anguilla rostrata American Shad Alosa sapidissima Appalachia Darter Percina gymnocephala Ashy Darter Etheostoma cinereum Atlantic Sturgeon Acipenser oxyrhynchus Banded Darter Etheostoma zonale Banded Drum Larimus fasciatus Banded Killifish Fundulus diaphanus Banded Sculpin Cottus carolinae Banded Sunfish Ennaecanthus obesus Bigeye Chub Hybopsis amblops Bigeye Jumprock Moxostoma ariommum Bigmouth Chub Nocomis platyrhynchus Black Bullhead Ameiurus melas Black Crappie Pomoxis nigromaculatus Blacktip Jumprock Moxostoma cervinum Black Redhorse Moxostoma duquesnei Black Sculpin Cottus baileyi Blackbanded Sunfish Enneacanthus chaetodon Blacknose Dace Rhinichthys atratulus Blackside Dace Chrosomus cumberlandensis Blackside Darter Percina maculata Blotched Chub Erimystax insignis Blotchside Logperch Percina burtoni Blue Catfish * Ictalurus furcatus * Blue Ridge Sculpin Cottus caeruleomentum Blueback Herring Alosa aestivalis Bluebreast Darter Etheostoma camurum Bluegill Lepomis macrochirus Bluehead Chub Nocomis leptocephalus Blueside Darter Etheostoma jessiae Bluespar Darter Etheostoma meadiae Bluespotted Sunfish Enneacanthus gloriosus Bluestone -
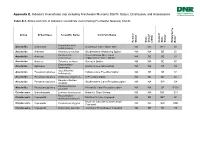
Status, Distribution, and Associations
Appendix E. Indiana’s Invertebrate (not including Freshwater Mussels) SGCN: Status, Distribution, and Associations Table E-1. Status and rank of Indiana’s invertebrate (not including Freshwater Mussels) SGCN. 1 2 2 Group Order/Class Scientific Name Common Name 3 Federal Status State Status (2005) Current State Status NatureServe Rank Hamohalacarus Arachnids Actinedida Donaldson Cave Water Mite NA NA SE* S1 subterraneus Arachnids Araneae Anahita punctulata Southeastern Wandering Spider NA NA SE S1 Porrhomma Cavernicolous Sheet-web Arachnids Araneae NA SE SE S1 cavernicola (Appalachian Cave) Spider Arachnids Araneae Talanites echinus Sac-web Spider NA NA SE S1 Erebomaster Arachnids Opiliones Golden Cave Harvestman NA NA ST S2 flavescens Apochthonius Arachnids Pseudoscorpiones Indiana Cave Pseudoscorpion NA SE SE S1 indianensis Arachnids Pseudoscorpiones Chthonius virginicus A Pseudoscorpion NA SE SE S1 Hesperochernes Arachnids Pseudoscorpiones Southeastern Cave Pseudoscorpion NA NA SW S4 mirabilis Kleptochthonius Arachnids Pseudoscorpiones Packard's Cave Pseudoscorpion NA NA SE S1S2 packardi Crustaceans Branchiopoda Lynceus brachyurus Holarctic Clam Shrimp NA NA SW* S1? Bryocamptus Crustaceans Copepoda Morrison's Cave Copepod NA SE SE* S1 morrisoni morrisoni Northern Cavefish (Commensal) Crustaceans Copepoda Cauloxenus stygius NA NA SW SNR Copepod Crustaceans Copepoda Diacyclops jeanneli Jeannel's Groundwater Copepod NA SE ST S2 1 2 2 Group Order/Class Scientific Name Common Name 3 Federal Status State Status (2005) Current State Status NatureServe Rank Megacyclops Crustaceans Copepoda Donaldson's Cave Copepod NA SE SE S1 donnaldsoni Crustaceans Malacostraca Caecidotea jordani Jordan's Groundwater Isopod NA SE SE S1 Crustaceans Malacostraca Caecidotea rotunda Northeastern (Frost) Cave Isopod NA SE SR S3 Indiana University Southeast Crustaceans Malacostraca Caecidotea teresae NA SE SE S1 Groundwater Isopod Crustaceans Malacostraca Crangonyx packardi Packard's Groundwater Amphipod NA SC SW S4 Crustaceans Malacostraca Crangonyx sp. -

Volume 2, Chapter 12-16: Terrestrial Insects: Holometabola-Mecoptera
Glime, J. M. 2017. Terrestrial Insects: Holometabola – Mecoptera. Chapt. 12-16. In: Glime, J. M. Bryophyte Ecology. Volume 2. 12-16-1 Bryological Interaction. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 19 July 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology2/>. CHAPTER 12-16 TERRESTRIAL INSECTS: HOLOMETABOLA – MECOPTERA TABLE OF CONTENTS MECOPTERA – SCORPIONFLIES ............................................................................................................... 12-16-2 Choristidae ................................................................................................................................................ 12-16-3 Boreidae .................................................................................................................................................... 12-16-3 Boreus ................................................................................................................................................ 12-16-4 Caurinus ............................................................................................................................................ 12-16-9 Hesperoboreus ................................................................................................................................. 12-16-20 Nannochoristidae .................................................................................................................................... 12-16-21 Panorpidae.............................................................................................................................................. -

The Distribution, Status & Conservation Needs of Canada's Endemic Species
Ours to Save The distribution, status & conservation needs of Canada’s endemic species June 4, 2020 Version 1.0 Ours to Save: The distribution, status & conservation needs of Canada’s endemic species Additional information and updates to the report can be found at the project website: natureconservancy.ca/ourstosave Citation Enns, Amie, Dan Kraus and Andrea Hebb. 2020. Ours to save: the distribution, status and conservation needs of Canada’s endemic species. NatureServe Canada and Nature Conservancy of Canada. Report prepared by Amie Enns (NatureServe Canada) and Dan Kraus (Nature Conservancy of Canada). Mapping and analysis by Andrea Hebb (Nature Conservancy of Canada). Cover photo credits (l-r): Wood Bison, canadianosprey, iNaturalist; Yukon Draba, Sean Blaney, iNaturalist; Salt Marsh Copper, Colin Jones, iNaturalist About NatureServe Canada A registered Canadian charity, NatureServe Canada and its network of Canadian Conservation Data Centres (CDCs) work together and with other government and non-government organizations to develop, manage, and distribute authoritative knowledge regarding Canada’s plants, animals, and ecosystems. NatureServe Canada and the Canadian CDCs are members of the international NatureServe Network, spanning over 80 CDCs in the Americas. NatureServe Canada is the Canadian affiliate of NatureServe, based in Arlington, Virginia, which provides scientific and technical support to the international network. About the Nature Conservancy of Canada The Nature Conservancy of Canada (NCC) works to protect our country’s most precious natural places. Proudly Canadian, we empower people to safeguard the lands and waters that sustain life. Since 1962, NCC and its partners have helped to protect 14 million hectares (35 million acres), coast to coast to coast.