Cook Inlet Oil & Gas Exploration, #AKG285100
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Freezeup Processes Along the Susitna River, Alaska
This document is copyrighted material. Permission for online posting was granted to Alaska Resources Library and Information Services (ARLIS) by the copyright holder. Permission to post was received via e-mail by Celia Rozen, Collection Development Coordinator on December 16, 2013, from Kenneth D. Reid, Executive Vice President, American Water Resources Association, through Christopher Estes, Chalk Board Enterprises, LLC. Five chapters of this symposium are directly relevant to the Susitna-Watana Hydroelectric Project, as they are about the Susitna Hydroelectric Project or about the Susitna River. This PDF file contains the following chapter: Freezeup processes along the Susitna River by Stephen R. Bredthauer and G. Carl Schoch ........................................................ pages 573-581 Assigned number: APA 4144 American Water Resources Association PROCEEDINGS of the Symposium: Cold Regions Hydrology UNIVERSITY OF ALASKA-FAIRBANKS, FAIRBANKS, ALASKA Edited by DOUGLASL.KANE Water Research Center Institute of Northern Engineering University of Alaska-Fairbanks Fairbanks, Alaska Co-Sponsored by UNIVERSITY OF ALASKA-FAIRBANKS FAIRBANKS, ALASKA AMERICAN SOCIETY OF CIVIL ENGINEERS fECHNICAL COUNCIL ON COLD REGIONS ENGINEERING NATIONAL SCIENCE FOUNDATION STATE OF ALASKA, ALASKA POWER AUTHORITY STATE OF ALASKA, DEPARTMENT OF NATURAL RESOURCES U.S. ARMY, COLD REGIONS RESEARCH AND ENGINEERING LABORATORY Host Section ALASKA SECTION OF THE AMERICAN WATER RESOURCES ASSOCIATION The American Water Resources Association wishes to express appreciation to the U.S. Army, Cold Regions Research and Engineering Laboratory, the Alaska Department of Natural Resources, and the Alaska Power Authority for their co-sponsorship of the publication of the proceedings. American Water Resources Association 5410 Grosvenor Lane, Suite 220 Bethesda, Maryland 20814 COLD REGIONS HYDROLOGY SYMPOSIUM JULY AMERICAN WATER RESOURCES ASSOCIATION 1986 FREEZEUP PROCESSES ALONG THE SUSIT:NA RIVER, ALASKA Stephen R. -

NSF 03-021, Arctic Research in the United States
This document has been archived. Home is Where the Habitat is An Ecosystem Foundation for Wildlife Distribution and Behavior This article was prepared The lands and near-shore waters of Alaska remaining from recent geomorphic activities such by Page Spencer, stretch from 48° to 68° north latitude and from 130° as glaciers, floods, and volcanic eruptions.* National Park Service, west to 175° east longitude. The immense size of Ecosystems in Alaska are spread out along Anchorage, Alaska; Alaska is frequently portrayed through its super- three major bioclimatic gradients, represented by Gregory Nowacki, USDA Forest Service; Michael imposition on the continental U.S., stretching from the factors of climate (temperature and precipita- Fleming, U.S. Geological Georgia to California and from Minnesota to tion), vegetation (forested to non-forested), and Survey; Terry Brock, Texas. Within Alaska’s broad geographic extent disturbance regime. When the 32 ecoregions are USDA Forest Service there are widely diverse ecosystems, including arrayed along these gradients, eight large group- (retired); and Torre Arctic deserts, rainforests, boreal forests, alpine ings, or ecological divisions, emerge. In this paper Jorgenson, ABR, Inc. tundra, and impenetrable shrub thickets. This land we describe the eight ecological divisions, with is shaped by storms and waves driven across 8000 details from their component ecoregions and rep- miles of the Pacific Ocean, by huge river systems, resentative photos. by wildfire and permafrost, by volcanoes in the Ecosystem structures and environmental Ring of Fire where the Pacific plate dives beneath processes largely dictate the distribution and the North American plate, by frequent earth- behavior of wildlife species. -

CMI Cook Inlet Surface Current Mapping Final Report
OCS Study MMS 2006-032 Final Report CODAR in Alaska Principal Investigator: Dave Musgrave, PhD Associate Professor School of Fisheries and Ocean Sciences University of Alaska Fairbanks P.O. Box 757220 Fairbanks, AK 99775-7220 (907) 474-7837 [email protected] CoAuthor: Hank Statscewich, MS School of Fisheries and Ocean Sciences University of Alaska Fairbanks P.O. Box 757220 Fairbanks, AK 99775-7220 (907) 474-5720 [email protected] June 2006 Contact information e-mail: [email protected] phone: 907.474.1811 fax: 907.474.1188 postal: Coastal Marine Institute School of Fisheries and Ocean Sciences P.O. Box 757220 University of Alaska Fairbanks Fairbanks, AK 99775-7220 ii Table of Contents List of Tables ................................................................................................................................. iv List of figures................................................................................................................................. iv Abstract........................................................................................................................................... v Introduction..................................................................................................................................... 1 Methods........................................................................................................................................... 2 Results............................................................................................................................................ -

Number of Fish Harvested (Thousands) 50
EASTSIDE SUSITNA UNIT Chulitna River Susitna River Talkeetna River WESTSIDE Deshka River SUSITNA UNIT TALKEETNA Yentna River KNIK ARM UNIT Skwentna River Willow Creek Matanuska River PALMER Little Knik River Susitna WEST COOK River INLET UNIT ANCHORAGE Beluga River Northern ALASKA Cook Inlet NORTH MAP 0 50 AREA MILES Figure 1.-Map of the Northern Cook Inlet sport fish management area. 2 450 Knik Arm Eastside Susitna Westside Susitna West Cook Inlet 400 350 300 250 200 150 11 100 50 Number of Angler-Days (Thousands) 1977 1978 1979 1980 1981 1982 1983 1984 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 Figure 2.-Angler-days of sport fishing effort expended by recreational anglers fishing Northern Cook Inlet Management Area waters, 1977-1999. 40 Mean 1999 35 30 25 20 15 10 5 13 Number of Angler-Days (Thousands) Marine Big Lake Knik River Finger Lake Other Lakes Wasilla Creek Other Streams Eklutna Tailrace Little Susitna River Cottonwood Creek Kepler Lk ComplexNancy Lk Complex Big Lake drainage streams Figure 3.-Mean number of angler-days per year of sport fishing effort expended at sites in the Knik Arm management unit, 1977-1998, and effort expended in 1999. 40 Mean 1999 35 30 25 20 15 16 10 5 Number of Angler-Days (Thousands) Lakes Little Willow Birch Creek Willow Creek Sheep CreekGoose Creek Caswell Creek Other Streams Kashwitna River Montana Creek Sunshine CreekTalkeetna River Figure 4.-Mean number of angler-days per year of sport fishing effort expended at sites in the Eastside Susitna River management unit, 1977-1998, and effort expended in 1999. -
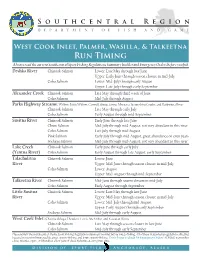
Run Timing Always Read the Current Southcentral Sport Fishing Regulations Summary Booklet and Emergency Orders Before You Fish
Southcentral Region Department of Fish and Game West Cook Inlet, Palmer, Wasilla, & Talkeetna Run Timing Always read the current Southcentral Sport Fishing Regulations Summary booklet and Emergency Orders before you fish. Deshka River Chinook Salmon Lower: Late May through late June Upper: Early June through season closure in mid-July Coho Salmon Lower: Mid-July through early August Upper: Late July through early September Alexander Creek Chinook Salmon Late May through third week of June Coho Salmon Mid-July through August Parks Highway Streams: Willow, Little Willow, Caswell, Sheep, Goose, Montana, & Sunshine Creeks, and Kashwitna River Chinook Salmon Late May through early July Coho Salmon Early August through mid-September Susitna River Chinook Salmon Early June through late June Chum Salmon Mid-July through mid-August, not very abundant in this river Coho Salmon Late July through mid-August Pink Salmon Early July through mid-August, great abundance on even years Sockeye Salmon Mid-July through mid-August, not very abundant in this river Lake Creek Chinook Salmon Early June through early July (Yentna River) Coho Salmon Early August through late August, early September Talachulitna Chinook Salmon Lower: June River Upper: Mid-June through season closure in mid-July Coho Salmon Lower: August Upper: Mid-August through mid-September Talkeetna River Chinook Salmon Mid-June through season closure in mid-July Coho Salmon Early August through September Little Susitna Chinook Salmon Lower: Late May through late June River Upper: Mid-June through season closure in mid-July Coho Salmon Lower: Mid-July through mid-August Upper: Early August through early September Sockeye Salmon Mid-July through early August West Cook Inlet: Chuitna, Beluga, Theodore, Lewis, McArthur, and Kustatan Rivers Chinook Salmon Late May through season closure in late June Coho Salmon Mid-July through early September Please review the Southcentral Alaska Sport Fishing Regulations Summary booklet before you go fishing. -

Nos Cook Inlet Operational Forecast System: Model Development and Hindcast Skill Assessment
NOAA Technical Report NOS CS 40 NOS COOK INLET OPERATIONAL FORECAST SYSTEM: MODEL DEVELOPMENT AND HINDCAST SKILL ASSESSMENT Silver Spring, Maryland September 2020 noaa National Oceanic and Atmospheric Administration U.S. DEPARTMENT OF COMMERCE National Ocean Service Coast Survey Development Laboratory Office of Coast Survey National Ocean Service National Oceanic and Atmospheric Administration U.S. Department of Commerce The Office of Coast Survey (OCS) is the Nation’s only official chartmaker. As the oldest United States scientific organization, dating from 1807, this office has a long history. Today it promotes safe navigation by managing the National Oceanic and Atmospheric Administration’s (NOAA) nautical chart and oceanographic data collection and information programs. There are four components of OCS: The Coast Survey Development Laboratory develops new and efficient techniques to accomplish Coast Survey missions and to produce new and improved products and services for the maritime community and other coastal users. The Marine Chart Division acquires marine navigational data to construct and maintain nautical charts, Coast Pilots, and related marine products for the United States. The Hydrographic Surveys Division directs programs for ship and shore-based hydrographic survey units and conducts general hydrographic survey operations. The Navigational Services Division is the focal point for Coast Survey customer service activities, concentrating predominately on charting issues, fast-response hydrographic surveys, and Coast Pilot -

Human Services Coordinated Transportation Plan for the Mat-Su Borough Area
Human Services Coordinated Transportation Plan For the Mat-Su Borough Area Phase III – 2011-2016 Final Draft January 2011 Table of Contents Introduction ..................................................................................................................... 3 Community Background .................................................................................................. 4 Coordinated Services Element ........................................................................................ 6 Coordination Working Group – Members (Table I) ................................................... 6 Inventory of Available Resources and Services (Description of Current Service / Public Transportation) .............................................................................................. 7 Description of Current Service / Other Transportation (Table II) .............................. 8 Assessment of Available Services – Public Transportation (Table III) ................... 13 Human Services Transportation Community Client Referral Form......................... 16 Population of Service Area: .................................................................................... 16 Annual Trip Destination Distribution – Current Service: ......................................... 19 Annual Trip Destination Distribution (Table V) ....................................................... 19 Vehicle Inventory .................................................................................................... 20 Needs Assessment ...................................................................................................... -

Geology of the Prince William Sound and Kenai Peninsula Region, Alaska
Geology of the Prince William Sound and Kenai Peninsula Region, Alaska Including the Kenai, Seldovia, Seward, Blying Sound, Cordova, and Middleton Island 1:250,000-scale quadrangles By Frederic H. Wilson and Chad P. Hults Pamphlet to accompany Scientific Investigations Map 3110 View looking east down Harriman Fiord at Serpentine Glacier and Mount Gilbert. (photograph by M.L. Miller) 2012 U.S. Department of the Interior U.S. Geological Survey Contents Abstract ..........................................................................................................................................................1 Introduction ....................................................................................................................................................1 Geographic, Physiographic, and Geologic Framework ..........................................................................1 Description of Map Units .............................................................................................................................3 Unconsolidated deposits ....................................................................................................................3 Surficial deposits ........................................................................................................................3 Rock Units West of the Border Ranges Fault System ....................................................................5 Bedded rocks ...............................................................................................................................5 -

Geological Characteristics in Cook Inlet Area, Alaska
SOCIE?I’YOF PETROLEUMENGINEERSOF AIME 6200 North CentralExpressway *R SPE 1588 Dallas,Texas 752C6 THIS IS A PREPRINT--- SUBJECTTO CORRECTION Geological Characteristics in Cook Inlet Area, Alaska Downloaded from http://onepetro.org/SPEATCE/proceedings-pdf/66FM/All-66FM/SPE-1588-MS/2087697/spe-1588-ms.pdf by guest on 25 September 2021 By ThomasE. Kelly,Jr. MemberAIYE, Mickl T. Halbouty,Houston,Tex. @ Copyright 19G6 Americsn Institute of Mining, Metallurgical and Petroleum Engineers, Inc. This paper was preparedfor the 41st AnnualFall Meetingof the Societyof PetroleumEngineers of AIME, to be held in Dallas,l?ex.,Oct. 2-5, 1966. permissionto copy is restrictedto an abstract of not more than 300 words. Illustrationsmay not be copied. The abstractshouldcontainconspicu- ous acknowledgmentof whereand by whom the paper is presented. Publicationelsewhereafter publica- tion in the JOURNALOF l?i’TROI.WJMTECHNOLOGYor the SOCIETYOF PETROLEUMENGINEERSJOURNALis usually grantedupon requestto the Editorof the appropriatejournalprovideciagreementto give propercredit is made. Discussionof this paper is invited. Three copiesof any discussionshouldbe sent to the Societyof PetroleumEngineersoffice. Such discussionmay be presentedat the abovemeetingand, with the paper,may be consideredfor publicationin one of the two WE magazines. v, The Cook Inlet basin is a narrow, Although the general characteristics elongate trough of Mesozoic and Ter- of the basin are fairly well known, tiary sediments located north of new information, as it is made avail- latitude 59° in south-central Alaska able will cause many revisions of the (Fig. 1). The basin covers approxi- stratigraphic and structural fabric mately 11,000 square miles of th~ before a complete geological picture northerripart of the Matanuska geo- is possible. -

Geologic Studies of the Lower Cook Inlet COST No.1 Wei Alaska Outer Contine Tal Shelf
Geologic Studies of the Lower Cook Inlet COST No.1 Wei Alaska Outer Contine tal Shelf GEOLOGIC STUDIES OF THE LOWER COOK INLET COST NO.1 WELL, ALASKA OUTER CONTINENTAL SHELF The ODECO Ocean Ranger, a semisubmersible drilling vessel, on location in lower Cook Inlet drilling the COST No.1 well. The view is southwest with Augustine Volcano, an active andesiticvolcano, on the horizon.ln the summerof1977 Atlantic Richfield, the operator, with 18 other participants from the petroleum industry drilled the well in Block No. 489 to a total depth of 3,775 .6 m. The well penetrated rocks that ranged in age from Late Jurassic to early Cenozoic. This well, drilled just before the opening of OCS Lease Sale No. Cl, confirmed among other things that Lower and Upper Cretaceous rocks are present under lower Cook Inlet and, as an additional bonus, penetrated several Upper Cre taceous sandstone bodies with petroleum reservoir potential. Geologic Studies of the Lower Cook Inlet COST No.1 Well, Alaska Outer Continental Shelf Leslie B. Magoon, Editor U.S. GEOLOGICAL SURVEY BULLETIN 1596 DEPARTMENT OF THE INTERIOR DONALD PAUL HODEL, Secretary U.S. GEOLOGICAL SURVEY Dallas l. Peck, Director UNITED STATES GOVERNMENT PRINTING OFFICE 1986 For sale by the Books and Open-File Reports Section U.S. Geological Survey Federal Center, Box 25425 Denver, CO 80225 Library of Congress Cataloging-in-Publication Data Geologic studies of the Lower Cook Inlet COST No. 1 well, Alaska Outer Continental Shelf. (U.S. Geological Survey Bulletin 1596) Includes bibliographies. Supt. of Docs. No.: I 19.3: 1596 1. -

On the Movement of Beluga Whales in Cook Inlet, Alaska
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Old Dominion University Old Dominion University ODU Digital Commons CCPO Publications Center for Coastal Physical Oceanography 12-2008 On the Movement of Beluga Whales in Cook Inlet, Alaska: Simulations of Tidal and Environmental Impacts Using a Hydrodynamic Inundation Model Tal Ezer Old Dominion University, [email protected] Roderick Hobbs Lie-Yauw Oey Follow this and additional works at: https://digitalcommons.odu.edu/ccpo_pubs Part of the Environmental Indicators and Impact Assessment Commons, Environmental Monitoring Commons, Marine Biology Commons, and the Oceanography Commons Repository Citation Ezer, Tal; Hobbs, Roderick; and Oey, Lie-Yauw, "On the Movement of Beluga Whales in Cook Inlet, Alaska: Simulations of Tidal and Environmental Impacts Using a Hydrodynamic Inundation Model" (2008). CCPO Publications. 117. https://digitalcommons.odu.edu/ccpo_pubs/117 Original Publication Citation Ezer, T., Hobbs, R., & Oey, L.Y. (2008). On the movement of beluga whales in Cook Inlet, Alaska: Simulations of tidal and environmental impacts using a hydrodynamic inundation model. Oceanography, 21(4), 186-195. doi: 10.5670/oceanog.2008.17 This Article is brought to you for free and open access by the Center for Coastal Physical Oceanography at ODU Digital Commons. It has been accepted for inclusion in CCPO Publications by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. or collective redistirbution of any portion of this article by photocopy machine, reposting, or other means is permitted only with the approval of The Oceanography Society. Send all correspondence to: [email protected] ofor Th e The to: [email protected] Oceanography approval Oceanography correspondence POall Box 1931, portionthe Send Society. -

Alaska M7.0 Earthquake Response
2018 M7.0 Anchorage, Alaska, Earthquake RESPONSE Date and Time: November 30, 2018, 8:29:29 am Location: N 61.323°, W 149.923° (7 miles north of Anchorage) Area of Effect: Strong to very strong shaking felt from northern Kenai Peninsula to Matanuska-Susitna Valley; light to moderate shaking felt throughout southcentral and interior Alaska. Initial earthquake followed 6 minutes later by M5.7 aftershock. Fatalities: 0 Damage: Power outages and gas leaks; damage to roads, railroads, and buildings; and closures of schools, businesses, and government offices throughout Anchorage bowl and Mat-Su Valley. Tsunami: Tsunami warnings were sent out within minutes of the earthquake. No tsunami waves Lateral spreading disrupted Vine Road near Wasilla. Many reported. failures of engineered materials occurred on or adjacent to water-saturated lowlands. (Photo credit: U.S. Geological Survey) Alaska Seismic Hazard Safety Commission Alaska Earthquake Center 2018 Cook Inlet Earthquake 1 Points to Ponder Proximity matters The Anchorage earthquake occurred 7 miles north of Anchorage and caused extensive damage, while the 2002 M7.9 Denali earthquake, which occurred about 170 miles north of Anchorage, caused no damage in the area. Anchorage population is approximately 325,00, Mat-Su 104,000 & Kenai Borough 50,000= 479,000 Entire state population is 710,000 Earthquake-resilient construction saves lives The population affected by the Anchorage earthquake generally reside in structures built in accordance with seismic building codes or using construction techniques that are resistant to earthquake shaking, and no lives were lost. Areas where codes were not enforced saw higher damage as a result Infrastructure is vulnerable to earthquake damage Construction codes for roads and embankments are not as successful as building codes for structures.