IUCN Directory of South Asian Protected Areas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Standards for Ruminant Sanctuaries
Global Federation of Animal Sanctuaries Standards For Ruminant Sanctuaries Version: April 2019 ©2012 Global Federation of Animal Sanctuaries Global Federation of Animal Sanctuaries – Standards for Ruminant Sanctuaries Table of Contents INTRODUCTION...................................................................................................................................... 1 GFAS PRINCIPLES ................................................................................................................................................... 1 ANIMALS COVERED BY THESE STANDARDS ............................................................................................................ 1 STANDARDS UPDATES ........................................................................................................................................... 2 RUMINANT STANDARDS ........................................................................................................................................ 2 RUMINANT HOUSING ........................................................................................................................... 2 H-1. Types of Space and Size ..................................................................................................................................... 2 H-2. Containment ...................................................................................................................................................... 5 H-3. Ground and Plantings ........................................................................................................................................ -

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

Low Res, 906 KB
Copyright: © 2011 Janzen and Bopage. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, Amphibian & Reptile Conservation 5(2):1-13. provided the original author and source are credited. The herpetofauna of a small and unprotected patch of tropical rainforest in Morningside, Sri Lanka 1,3PETER JANZEN AND 2MALAKA BOPAGE 1Rheinallee 13, 47119 Duisburg, GERMANY 2Biodiversity Education & Exploration Society (BEES) 63/c Wackvella road Galle 80000, SRI LANKA Abstract.—Morningside is an exceptional area in Sri Lanka with highly endemic herpetofauna. How- ever, this relictual forest area lies inside a tea plantation and is mostly lacking conservation protec- tion. Species inventories of remaining rainforest patches are currently incomplete, and information about the behavior and ecology of the herpetofauna of Morningside is poorly known. In our survey, we identified 13 amphibian species and recorded an additional two species that could not be identi- fied with existing keys. We determined 11 reptile species from this patch of forest, and another un- identifiedCnemaspis gecko was recorded. We did not assess the herpetofauna outside of this forest patch. Some species are described for the first time in Morningside, suggesting a wider distribution in Sri Lanka. We also document a call from a male Pseudophilautus cavirostris for the first time. Perspectives for future surveys are given. Key words. Survey, Morningside, Sri Lanka, herpetofauna, conservation, Pseudophilautus cavirostris Citation: Jansen, P. and Bopage, M. 2011. The herpetofauna of a small and unprotected patch of tropical rainforest in Morningside, Sri Lanka. -

Downloadable As a PDF
Frontlines Dispatches - Vol II, Number 7, July 2020 Page 1 DEDICATED TO THE WORLD’S CUSTODIANS OF WILD SPACES & WILDLIFE North & South America .......................................................................................................................2 Europe .........................................................................................................................................................4 Africa ............................................................................................................................................................6 Asia ................................................................................................................................................................8 World ...........................................................................................................................................................10 A World That Values the Conservation and Livelihood Benefits of Sustainable Wildlife Utilization Frontlines Dispatches - Vol II, Number 7, July 2020 Page 2 North & South America California’s Academy of Sciences BigPicture Photo Competition celebrates some of the world’s most striking nature and conservation images in hopes of inspiring viewers to protect and conserve the diversity of life on Earth. Biographic presents this year’s 12 winning photos. High-tech help for Smokey the Bear. A team from Michigan State University has built a forest- fire detection and alarm system powered by the movement of tree branches in the wind. As reported -

Malawi Trip Report 12Th to 28Th September 2014
Malawi Trip Report 12th to 28th September 2014 Bohm’s Bee-eater by Keith Valentine Trip Report compiled by Tour Leader: Keith Valentine RBT Malawi Trip Report September 2014 2 Top 10 Birds: 1. Scarlet-tufted Sunbird 2. Pel’s Fishing Owl 3. Lesser Seedcracker 4. Thyolo Alethe 5. White-winged Apalis 6. Racket-tailed Roller 7. Blue Swallow 8. Bohm’s Flycatcher 9. Babbling Starling 10. Bohm’s Bee-eater/Yellow-throated Apalis Top 5 Mammals: 1. African Civet 2. Four-toed Elephant Shrew 3. Sable Antelope 4. Bush Pig 5. Side-striped Jackal/Greater Galago/Roan Antelope/Blotched Genet Trip Summary This was our first ever fully comprehensive tour to Malawi and was quite simply a fantastic experience in all respects. For starters, many of the accommodations are of excellent quality and are also situated in prime birding locations with a large number of the area’s major birding targets found in close proximity. The food is generally very good and the stores and lodges are for the most part stocked with decent beer and a fair selection of South African wine. However, it is the habitat diversity that is largely what makes Malawi so good from a birding point of view. Even though it is a small country, this good variety of habitat, and infrastructure that allows access to these key zones, insures that the list of specials is long and attractive. Our tour was extremely successful in locating the vast majority of the region’s most wanted birds and highlights included Red-winged Francolin, White-backed Night Heron, African Cuckoo-Hawk, Western Banded Snake -

29Th 2019-Uganda
AVIAN SAFARIS 23 DAY UGANDA BIRDING AND NATURE TOUR ITINERARY Date: July 7 July 29, 2019 Tour Leader: Crammy Wanyama Trip Report and all photos by Crammy Wanyama Black-headed Gonolek a member of the Bush-shrikes family Day 1 – July 7, 2019: Beginning of the tour This tour had uneven arrivals. Two members arrived two days earlier and the six that came in on the night before July 7th, stayed longer; therefore, we had a pre and post- tour to Mabira Forest. For today, we all teamed up and had lunch at our accommodation for the next two nights. This facility has some of the most beautiful gardens around Entebbe; we decided to spend the rest of the afternoon here watching all the birds you would not expect to find around a city garden. Some fascinating ones like the Black-headed Gonolek nested in the garden, White-browed Robin-Chat too did. The trees that surrounded us offered excellent patching spots for the African Hobby. Here we had a Falco patching out in the open for over forty minutes! Superb looks at a Red-chested and Scarlet-chested Sunbirds. The gardens' birdbath attracted African Thrush that reminded the American birders of their American Robin, Yellow- throated Greenbul. Still looking in the trees, we were able to see African Grey Woodpeckers, both Meyer's and Grey Parrot, a pair of Red-headed Lovebirds. While walking around the facility, we got good looks at a flying Shikra and spent ample time with Ross's Turaco that flew back and forth. We had a very lovely Yellow-fronted Tinkerbird on the power lines, Green-backed Camaroptera, a very well sunlit Avian Safaris: Email: [email protected] Website: http://www.aviansafaris.com AVIAN SAFARIS Spectacled Weaver, was added on the Village and Baglafecht Weavers that we had seen earlier and many more. -

Dwarf Mistletoes: Biology, Pathology, and Systematics
This file was created by scanning the printed publication. Errors identified by the software have been corrected; however, some errors may remain. CHAPTER 10 Anatomy of the Dwarf Mistletoe Shoot System Carol A. Wilson and Clyde L. Calvin * In this chapter, we present an overview of the Morphology of Shoots structure of the Arceuthobium shoot system. Anatomical examination reveals that dwarf mistletoes Arceuthobium does not produce shoots immedi are indeed well adapted to a parasitic habit. An exten ately after germination. The endophytic system first sive endophytic system (see chapter 11) interacts develops within the host branch. Oftentimes, the only physiologically with the host to obtain needed evidence of infection is swelling of the tissues near the resources (water, minerals, and photosynthates); and infection site (Scharpf 1967). After 1 to 3 years, the first the shoots provide regulatory and reproductive func shoots are produced (table 2.1). All shoots arise from tions. Beyond specialization of their morphology (Le., the endophytic system and thus are root-borne shoots their leaves are reduced to scales), the dwarf mistle (Groff and Kaplan 1988). In emerging shoots, the toes also show peculiarities of their structure that leaves of adjacent nodes overlap and conceal the stem. reflect their phylogenetic relationships with other As the internodes elongate, stem segments become mistletoes and illustrate a high degree of specialization visible; but the shoot apex remains tightly enclosed by for the parasitic habit. From Arceuthobium globosum, newly developing leaf primordia (fig. 10.lA). Two the largest described species with shoots 70 cm tall oppositely arranged leaves, joined at their bases, occur and 5 cm in diameter, toA. -

Light House Project at Agartala, Tripura
Light House Project at Agartala, Tripura Ministry of Housing & Urban Affairs Government of India LIGHT HOUSE PROJECT AT AGARTALA, TRIPURA 3D View of the Project 1 pmay-urban.gov.in ghtc-india.gov.in PMAYUrban pmayurban PMAY Urban [email protected] Ministry of Housing & Urban Affairs, Govt. of India The country is going to get a new technology to build houses “ for the poor and the middle class. In technical parlance, you call it the Light House Project. I believe these six projects are really like light towers. These six light house projects would give a new direction to the housing construction in the country. The coming together of states from the east-west, north-south and every region of the country is further strengthening our sense of cooperative federalism. These light house projects will be constructed through modern technology and innovative processes. This will reduce the construction time and prepare the more resilient, affordable and comfortable homes for the poor. In a way, these projects will be incubation centres and our planners, architects, engineers and students will be able to learn and experiment with new technology. ” Narendra Modi Prime Minister of India 1.1.2021 2 Light House Project at Agartala, Tripura 1. Background The Ministry of Housing and Urban Affairs (MoHUA) is implementing Pradhan Mantri Awas Yojana-Urban (PMAY-U) Mission, one of the largest public housing programs in the world, with a goal of providing all weather pucca houses to all eligible urban families by 2022. Against an assessed demand of 1.12 crore houses, so far over 1.08 crore have been sanctioned; out of this over 72 lakh have been grounded for construction and nearly 42 lakh have been completed and delivered to the beneficiaries. -

Impact of Covid-19 in Nagaland, North East India
International Journal of Economics and Financial Issues ARF INDIA Vol. 2, No. 1-2, 2021, pp. 81-92 Academic Open Access Publishing www.arfjournals.com © ARF India. All Right Reserved IMPACT OF COVID-19 IN NAGALAND, NORTH EAST INDIA B. Imnawapang Longkumer Assistant Professor, Department of Economics, Fazl Ali College, Mokokchung, Nagaland E-mail: [email protected] ABSTRACT: The outbreak of the Covid-19 pandemic has resulted in an unprecedented shock to the world economy. The government of India, Article History under Prime Minister Narendra Modi declared a nationwide Lockdown Received : 16 February 2021 on 24th March 2020 and due to the prolonged lockdown, the state of Revised : 23 February 2021 Nagaland along with the rest of the country is facing many difficulties Accepted : 9 March 2021 and challenges. With the first reported case of COVID-19 from Wuhan, Published : 3 May 2021 China in December 2019, and as of 15th November 2020, globally 53.7 million confirmed cases and 1.3 million deaths have been reported as Key words per World Health Organization (WHO), with India reporting 9.14 COVID-19, Lockdown, million cases, 8.6 million recovered, 134 thousand deaths and Nagaland Nagaland, Technology, Impact. with 10,674 cases, 9242 recovered, 57 deaths. Manipur was the first state in Northeast India to have detected with COVID-19 case as on 24th March 2020, with a 23 years old student returnee from UK, while Nagaland was the last of the northeastern States after Sikkim to report COVID-19 positive cases on April 12, 2020. Since Nagaland has no big or very few industries the major impact is on tourism, handicrafts and handloom industry, agriculture and rural economy and small business etc, especially festival like Hornbill festival where the states earns around 40-50 crores on revenue. -

Northeast States
©Lonely Planet Publications Pty Ltd Northeast States Includes ¨ Why Go? Assam .............561 Thrown across the farthest reaches of India, obscured from Guwahati ...........561 the greater world by ageless forests and formidable moun- Kaziranga tain ranges, the Northeast States are one of Asia’s last great National Park .......567 natural and anthropological sanctuaries. Sharing borders Arunachal Pradesh ...572 with Bhutan, Tibet, Myanmar (Burma) and Bangladesh, these remote frontiers are a region of rugged beauty, and Nagaland ...........579 a collision zone of tribal cultures, climates, landscapes and Kohima .............579 peoples. In this wonderland for adventurers, glacial Hima- Manipur ........... 583 layan rivers spill onto Assam’s vast floodplains, faith moves Mizoram ........... 584 mountains on the perilous pilgrimage to Tawang, rhinos Tripura ............ 586 graze in Kaziranga’s swampy grasslands and former head- hunters slowly embrace modernity in their ancestral long- Agartala ........... 586 houses in Nagaland. Meghalaya ......... 588 Of course, it’s not all smooth sailing in these faraway Shillong ........... 588 states, and there’s a horde of obstacles to battle along the way (bad roads, poor infrastructure and rebel armies, to name a few). Only those with a taste for raw adventure need apply. Best Places to Eat ¨ Paradise (p563) When to Go ¨ Luxmi Kitchen (p584) Assam (Guwahati) ¨ Moti Mahal (p571) °C/°F Te mp Rainfall inches/mm 40/104 32/800 ¨ Maihang (p568) 24/600 ¨ Trattoria (p591) 20/68 16/400 0/32 Best Places 8/200 -20/-4 0 to Sleep J FDM A M J J A S O N ¨ Diphlu River Lodge (p568) Mar The best Oct A time for Dec Fierce Naga ¨ Puroni Bheti (p569) season for dazzling Hima- warriors in ethnic rhino-spotting layan vistas and regalia assemble ¨ Ri Kynjai (p591) in Kaziranga. -

Microhabitat Preferences and Associated Behavior Patterns Of
Journal of Entomology and Zoology Studies 2019; 7(4): 924-928 E-ISSN: 2320-7078 P-ISSN: 2349-6800 Microhabitat preferences and associated behavior JEZS 2019; 7(4): 924-928 © 2019 JEZS patterns of endemic pigmy lizard: Cophotis Received: 13-05-2019 Accepted: 16-06-2019 ceylanica in Horton plains, Sri Lanka WLR Keerthirathna Department of Zoology, University of Sri WLR Keerthirathna and WAD Mahaulpatha Jayewardenepura, Sri Lanka Abstract WAD Mahaulpatha The pigmy lizard (Cophotis ceylanica) is an endangered and rare lizard species endemic to Sri Lanka, yet Department of Zoology, University of Sri no studies exist on its microhabitat preferences. Therefore, the present study was carried out in the Cloud Jayewardenepura, Sri Lanka Forests of the Horton Plains National Park (HPNP) with the aim of addressing this knowledge gap in their ecology. Microhabitat variables were measured placing 1x1 m quadrates marking the point of each lizard sighting as the center and microhabitat details including perch plant characteristics, soil characteristics and environmental parameters were recorded. Highest number of individuals were seen on Sarcococca brevifolia (1.167±0.937) plant species. Total of 78.13%, C. ceylanica were observed perching on branches rather than on trunks or leaves. Highest percentage of pigmy lizards (48.87%) was recorded in the branches where the moss cover was between 50% - 75% and the lowest of 12.50% was recorded where the moss cover was less than 25%. Highest percentage of 71.88% of C. ceylanica were recorded perching in the height category of 2-3 m of perching plants. No individuals were recorded up to 1m from ground level. -
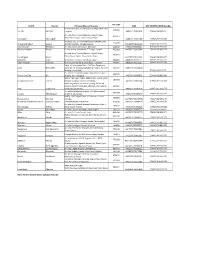
GASTN NO SEND to UPLOAD on AI WEB SITE DT 11 OCT 17-A.Xlsm
Pin Code STATE District Principal Place of Business ARN GST REGISTRATION Number Sri Guru Ram Dass Jee International Airport,Aiatsl Gsd 143101 Punjab Amritsar Complex AA0307170452494 03AAECA6186G1ZT Air India, New Terminal Bhawan, Airport Raipur, 492015 Raipur Mana Airport, Mana Camp, Raipur Chandigarh Chandigarh AA040717000724H 04AAECA6186G1ZR AIR INDIA LTD., STATION MANAGER, KANGRA CIVIL 176209 Himachal Pradesh Kangra AIRPORT GAGGAL , DHARAMSHALA AA020717002414Q 02AAECA6186G1ZV Uttarakhand Dehradun Air India, Jolly Grant Airport, Dehradun 248140 AA050717003135L 05AAECA6186G1ZP Madhya Pradesh Bhopal Airlines House, Bhadbhada, T.T. Nagar, Bhopal 462003 AA230717004056K 23AAECA6186G1ZR Air India, New Terminal Bhawan, Airport Raipur, 492015 Raipur Mana Airport, Mana Camp, Raipur Chhatisgarh Raipur AA220717001226O 22AAECA6186G1ZT Rajasthan Jaipur Nehru Place Complex, Tank Road, Jaipur 302015 AA080717025111J 08AAECA6186G1ZJ Uttar Pradesh Lucknow 9, Rani Laxmi Bai Marg, China Bazar Lucknow 226001 AA090717013013J 09AAECA6186G1ZH Deptt. Of Information Tech. 2Nd Floor, Telephone Delhi New Delhi Exchange, Air India Gsd Complex, Igi Airport, Terminal 110037 AA070717004291G Building-2, New Delhi 07AAECA6186G1ZL Air India Ltd, Station Manager, Nagoa Road, Airport 362571 Daman And Diu Diu Building, Diu - Junagadh AA2506170005893 25AAECA6186G1ZN Station Manager Indian Airlines Ltd., Civil Airport, 180003 Jammu & Kashmir Jammu Satwari, Jammu, Jammu & Kashmir, AA010717008534I 01AAECA6186G1ZX New Integrated International Building, DABOLIM AIRPOT, Dabolim