Development and Evaluation of Automated Radar Systems for Monitoring and Characterising Echoes from Insect Targets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Locusts in Queensland
LOCUSTS Locusts in Queensland PEST STATUS REVIEW SERIES – LAND PROTECTION by C.S. Walton L. Hardwick J. Hanson Acknowledgements The authors wish to thank the many people who provided information for this assessment. Clyde McGaw, Kevin Strong and David Hunter, from the Australian Plague Locust Commission, are also thanked for the editorial review of drafts of the document. Cover design: Sonia Jordan Photographic credits: Natural Resources and Mines staff ISBN 0 7345 2453 6 QNRM03033 Published by the Department of Natural Resources and Mines, Qld. February 2003 Information in this document may be copied for personal use or published for educational purposes, provided that any extracts are fully acknowledged. Land Protection Department of Natural Resources and Mines GPO Box 2454, Brisbane Q 4000 #16401 02/03 Contents 1.0 Summary ................................................................................................................... 1 2.0 Taxonomy.................................................................................................................. 2 3.0 History ....................................................................................................................... 3 3.1 Outbreaks across Australia ........................................................................................ 3 3.2 Outbreaks in Queensland........................................................................................... 3 4.0 Current and predicted distribution ........................................................................ -

NON-REGULATED PESTS (Non-Actionable)
Import Health Standard Commodity Sub-class: Fresh Fruit/Vegetables Grape, Vitis vinifera from Australia ISSUED Issued pursuant to Section 22 of the Biosecurity Act 1993 Date Issued: 20 December 2000 1 NEW ZEALAND NATIONAL PLANT PROTECTION ORGANISATION The official contact point in New Zealand for overseas NPPOs is the Ministry for Primary Industries (MPI). All communication pertaining to this import health standard should be addressed to: Manager, Import and Export Plants Ministry for Primary Industries PO Box 2526 Wellington NEW ZEALAND Fax: 64-4-894 0662 E-mail: [email protected] http://www.mpi.govt.nz 2 GENERAL CONDITIONS FOR ALL PLANT PRODUCTS All plants and plant products are PROHIBITED entry into New Zealand, unless an import health standard has been issued in accordance with Section 22 of the Biosecurity Act 1993. Should prohibited plants or plant products be intercepted by MPI, the importer will be offered the option of reshipment or destruction of the consignment. The national plant protection organisation of the exporting country is requested to inform MPI of any change in its address. The national plant protection organisation of the exporting country is required to inform MPI of any newly recorded organisms which may infest/infect any commodity approved for export to New Zealand. Pursuant to the Hazardous Substances and New Organisms Act 1996, proposals for the deliberate introduction of new organisms (including genetically modified organisms) as defined by the Act should be referred to: IHS Fresh Fruit/Vegetables. Grape, Vitis vinifera from Australia. (Biosecurity Act 1993) ISSUED: 20 December 2000 Page 1 of 16 Environmental Protection Authority Private Bag 63002 Wellington 6140 NEW ZEALAND Or [email protected],nz Note: In order to meet the Environmental Protection Authority requirements the scientific name (i.e. -

Biological Surveys at Hunsbury Hill Country Park 2018
FRIENDS OF WEST HUNSBURY PARKS BIOLOGICAL SURVEYS AT HUNSBURY HILL COUNTRY PARK 2018 Ryan Clark Northamptonshire Biodiversity Records Centre April 2019 Northamptonshire Biodiversity Records Centre Introduction Biological records tell us which species are present on sites and are essential in informing the conservation and management of wildlife. In 2018, the Northamptonshire Biodiversity Records Centre ran a number of events to encourage biological recording at Hunsbury Hill Fort as part of the Friends of West Hunsbury Park’s project, which is supported by the National Lottery Heritage Fund. Hunsbury Hill Country Park is designated as a Local Wildlife Site (LWS). There are approximately 700 Local Wildlife Sites in Northamptonshire. Local Wildlife Sites create a network of areas, which are important as refuges for wildlife or wildlife corridors. Hunsbury Hill Country Park was designated as a LWS in 1992 for its woodland flora and the variety of habitats that the site possesses. The site also has a Local Geological Site (LGS) which highlights the importance of this site for its geology as well as biodiversity. This will be surveyed by the local geological group in due course. Hunsbury Hill Country Park Local Wildlife Site Boundary 1 Northamptonshire Biodiversity Records Centre (NBRC) supports the recording, curation and sharing of quality verified environmental information for sound decision-making. We hold nearly a million biological records covering a variety of different species groups. Before the start of this project, we looked to see which species had been recorded at the site. We were surprised to find that the only records we have for the site have come from Local Wildlife Site Surveys, which assess the quality of the site and focus on vascular plants, with some casual observations of other species noted too. -

Origins of Six Species of Butterflies Migrating Through Northeastern
diversity Article Origins of Six Species of Butterflies Migrating through Northeastern Mexico: New Insights from Stable Isotope (δ2H) Analyses and a Call for Documenting Butterfly Migrations Keith A. Hobson 1,2,*, Jackson W. Kusack 2 and Blanca X. Mora-Alvarez 2 1 Environment and Climate Change Canada, 11 Innovation Blvd., Saskatoon, SK S7N 0H3, Canada 2 Department of Biology, University of Western Ontario, Ontario, ON N6A 5B7, Canada; [email protected] (J.W.K.); [email protected] (B.X.M.-A.) * Correspondence: [email protected] Abstract: Determining migratory connectivity within and among diverse taxa is crucial to their conservation. Insect migrations involve millions of individuals and are often spectacular. However, in general, virtually nothing is known about their structure. With anthropogenically induced global change, we risk losing most of these migrations before they are even described. We used stable hydrogen isotope (δ2H) measurements of wings of seven species of butterflies (Libytheana carinenta, Danaus gilippus, Phoebis sennae, Asterocampa leilia, Euptoieta claudia, Euptoieta hegesia, and Zerene cesonia) salvaged as roadkill when migrating in fall through a narrow bottleneck in northeast Mexico. These data were used to depict the probabilistic origins in North America of six species, excluding the largely local E. hegesia. We determined evidence for long-distance migration in four species (L. carinenta, E. claudia, D. glippus, Z. cesonia) and present evidence for panmixia (Z. cesonia), chain (Libytheana Citation: Hobson, K.A.; Kusack, J.W.; Mora-Alvarez, B.X. Origins of Six carinenta), and leapfrog (Danaus gilippus) migrations in three species. Our investigation underlines Species of Butterflies Migrating the utility of the stable isotope approach to quickly establish migratory origins and connectivity in through Northeastern Mexico: New butterflies and other insect taxa, especially if they can be sampled at migratory bottlenecks. -

20 Taxonomic Significance of Aedeagus in the Classification Of
International Journal of Entomology Research International Journal of Entomology Research ISSN: 2455-4758; Impact Factor: RJIF 5.24 www.entomologyjournals.com Volume 1; Issue 7; November 2016; Page No. 20-31 Taxonomic significance of aedeagus in the classification of Indian Acrididae (Orthoptera: Acridoidea) Shahnila USMANI, Mohd. Kamil Usmani, Mohammad AMIR Section of Entomology, Department of Zoology, Aligarh Muslim University, Aligarh, Uttar Pradesh, India Abstract Comparative study of aedeagus is made in one hundred and two species of grasshoppers representing fifty-nine genera belonging to the family Acrididae. Its taxonomic significance is shown. Divided, undivided or flexured conditions of aedeagus is taken as familial character. Apical valve of aedeagus longer or shorter than basal valve is considered as generic character. Shape of apical and basal valves is suggested as specific character. Keywords: 1. Introduction done in clove oil. The aedeagus was mounted in Canada The aedeagus is a main intromittent organ consisting of a pair balsam on a cavity slide under 22mm square cover glass. of basal and apical valves. The basal valves are lying above the Drawings were made with the help of Camera lucida. spermatophore sac and connected by the flexure with the long curved apical valves which are normally concealed under the 3. Description of Aedeagus membranous pallium. During the course of copulation it is Subfamily Acridinae inserted between ventral ovipositor valves of the female into 1. Truxalis eximia Eichwald, 1830 (Fig. 1 A) vagina and its tip reaches the spermathecal duct. Dirsh & Aedeagus flexured, apical valve long and narrow, slightly Uvarov (1953) [2] studied apical valves of penis in three species curved, apex obtusely pointed, slightly narrower and shorter of Anacridium. -

JEB Classics
JEB Classics 177 THE ORIGIN OF INSECT George Newport had reported that there is JEB Classics is an occasional THERMOREGULATORY a correlation between activity and elevated column, featuring historic body temperature in a moth, a bumblebee, publications from The Journal of STUDIES and a beetle (Newport, 1837). After the Experimental Biology. These subject remained fallow for the following articles, written by modern experts 60 years the Russian physicist Perfirij J. in the field, discuss each classic Bachmetjev resurrected the subject when paper’s impact on the field of he identified the same correlation in insects biology and their own work. A just before the end of the 19th Century PDF of the original paper is (Bachmetjev, 1899). Similarly, Heinz available from the JEB Archive Dotterweich showed specifically that the (http://jeb.biologists.org/). rise in thoracic temperature of sphinx moths is related to the insects’ flight preparations (Dotterweich, 1928). In Krogh’s own laboratory in Denmark, Marius Nielsen showed that human body temperature also rises during strenuous activity, and is then regulated at a high level corresponding to work output (Nielsen, 1938). Referencing these early, possibly forgotten, classical studies in Krogh and Zeuthen’s 1941 paper brought the neglected topic of thermoregulation to the forefront of the then hot field of respiratory physiology. Prior to Krogh and Zeuthen’s work, reports Bernd Heinrich writes about August Krogh of insect thermoregulation were mainly and Eric Zeuthen’s 1941 classic paper on s descriptive. However, their 1941 paper was insect thermoregulation entitled ‘The the first to attempt to crack the proverbial mechanism of flight preparation in some black box of the underlying physiological insects’. -

197 Section 9 Sunflower (Helianthus
SECTION 9 SUNFLOWER (HELIANTHUS ANNUUS L.) 1. Taxonomy of the Genus Helianthus, Natural Habitat and Origins of the Cultivated Sunflower A. Taxonomy of the genus Helianthus The sunflower belongs to the genus Helianthus in the Composite family (Asterales order), which includes species with very diverse morphologies (herbs, shrubs, lianas, etc.). The genus Helianthus belongs to the Heliantheae tribe. This includes approximately 50 species originating in North and Central America. The basis for the botanical classification of the genus Helianthus was proposed by Heiser et al. (1969) and refined subsequently using new phenological, cladistic and biosystematic methods, (Robinson, 1979; Anashchenko, 1974, 1979; Schilling and Heiser, 1981) or molecular markers (Sossey-Alaoui et al., 1998). This approach splits Helianthus into four sections: Helianthus, Agrestes, Ciliares and Atrorubens. This classification is set out in Table 1.18. Section Helianthus This section comprises 12 species, including H. annuus, the cultivated sunflower. These species, which are diploid (2n = 34), are interfertile and annual in almost all cases. For the majority, the natural distribution is central and western North America. They are generally well adapted to dry or even arid areas and sandy soils. The widespread H. annuus L. species includes (Heiser et al., 1969) plants cultivated for seed or fodder referred to as H. annuus var. macrocarpus (D.C), or cultivated for ornament (H. annuus subsp. annuus), and uncultivated wild and weedy plants (H. annuus subsp. lenticularis, H. annuus subsp. Texanus, etc.). Leaves of these species are usually alternate, ovoid and with a long petiole. Flower heads, or capitula, consist of tubular and ligulate florets, which may be deep purple, red or yellow. -

Challenges and Prospects in the Telemetry of Insects
Biol. Rev. (2014), 89, pp. 511–530. 511 doi: 10.1111/brv.12065 Challenges and prospects in the telemetry of insects W. Daniel Kissling1,2,∗, David E. Pattemore3 and Melanie Hagen4 1Ecoinformatics & Biodiversity, Department of Bioscience, Aarhus University, Ny Munkegade 114, DK-08000 Aarhus C, Denmark 2Institute for Biodiversity and Ecosystem Dynamics (IBED), University of Amsterdam, PO Box 94248, 1090 GE Amsterdam, The Netherlands 3The New Zealand Institute for Plant & Food Research Limited, Private Bag 3230, Waikato Mail Centre, Hamilton 3240, New Zealand 4Genetics & Ecology, Department of Bioscience, Aarhus University, Ny Munkegade 114, DK-08000 Aarhus C, Denmark ABSTRACT Radio telemetry has been widely used to study the space use and movement behaviour of vertebrates, but transmitter sizes have only recently become small enough to allow tracking of insects under natural field conditions. Here, we review the available literature on insect telemetry using active (battery-powered) radio transmitters and compare this technology to harmonic radar and radio frequency identification (RFID) which use passive tags (i.e. without a battery). The first radio telemetry studies with insects were published in the late 1980s, and subsequent studies have addressed aspects of insect ecology, behaviour and evolution. Most insect telemetry studies have focused on habitat use and movement, including quantification of movement paths, home range sizes, habitat selection, and movement distances. Fewer studies have addressed foraging behaviour, activity patterns, migratory strategies, or evolutionary aspects. The majority of radio telemetry studies have been conducted outside the tropics, usually with beetles (Coleoptera) and crickets (Orthoptera), but bees (Hymenoptera), dobsonflies (Megaloptera), and dragonflies (Odonata) have also been radio-tracked. -

Morphology and Development of Oocyte and Follicle Resorption Bodies in the Lubber Grasshopper, Romalea Microptera (Beauvois)
S.V. SUNDBERG, M.H. LUONG-SKOVMANDJournal of Orthoptera AND D.W. Research, WHITMAN June 2001, 10 (1): 39-5139 Morphology and development of oocyte and follicle resorption bodies in the Lubber grasshopper, Romalea microptera (Beauvois) STEVEN V. SUNDBERG, MY HANH LUONG-SKOVMAND AND DOUGLAS W. WHITMAN [SVS, DWW] Behavior, Ecology, Evolution, & Systematic Section, 4120 Department of Biological Sciences, Illinois State University, Normal, IL 61790-4120, USA e-mail: [email protected] [MHLS] CIRAD, Centre de Cooperation Internationale en Recherche Agronomique pour le Developpement (Prifas) BP 5035 - 34032 Montpellier cedex 1 - France e-mail: [email protected] Abstract We describe the development and appearance of Follicle Resorption ovocyte, produisent un corps de régression de couleur jaune orangé. Bodies (FRBs) and Oocyte Resorption Bodies (ORBs) in the Le nombre de traces de ponte est égal au nombre d’oeufs pondus et grasshopper Romalea microptera (= guttata), and demonstrate that le nombre de corps de régression au nombre d’ovocytes ayant these structures can be used to determine the past ovipositional and régressé. Les R. microptera en bonne santé et nourris en abondance environmental history of females. In R. microptera, one resorption résorbent environ le quart de leur ovocytes en croissance. La privation body is deposited at the base of each ovariole following each de nourriture ou le stress physiologique entrainent une gonotropic cycle. These structures are semi-permanent, and remain augmentation du taux de régression ovocytaire et par conséquent distinct for at least 8 wks and two additional ovipositions. Ovarioles du nombre de corps de régression (ORB). Si l’on compte les traces that ovulate a mature, healthy oocyte, produce a cream-colored de ponte et les corps de régression dans chaque ovariole, on obtient FRB. -

Climate and Rice Insects
367 Climate and rice insects R. Kisimoto and V. A. Dyck SUMMARY limatic factors such as temperature, relative humidity, rainfall, and mass air C movements may affect the distribution, development, survival, behavior. migration, reproduction, population dynamics, and outbreaks of insect pests of rice. These factors usually act in a density-independent manner, influencing insects to a greater or lesser extent depending on the situation and the insect species. Temperature conditions set the basic limits to insect distribution, and ex- amples are given of distribution patterns in northeastern Asia in relation to temperature extremes and accumulation. Diapause is common in insects indigenous to the temperate regions, but in the tropics, diapause does not usually occur. it is induced by short photoperiod, low temperature, and sometimes the quality of the food to enable the insect to overwinter. Population outbreaks have been related to various climatic factors, such as previous winter temperature, temperature of the current season, and rainfall. High temperature and low rainfall can cause a severe stem borer infestation. Rainfall is important for population increase of the oriental armyworm, and of rice green leafhoppers and rice gall midges in the tropics. The cause of migrations of Mythimna separata (Walker) has been traced to wind direction and population growth patterns in different climatic areas of China. It is believed that Sogatella furcifera (Horvath) and Nilaparvata lugens (Sta1) migrate passively each year into Japan and Korea from more southerly areas. Probably these insects spread out annually from tropical to subtropical zones where they multiply and then migrate to temperate zones. Considerable knowledge is available on the effects of climate on rice insects through controlled environment studies and careful observations and statistical comparisons of events in the field, However, much more conclusive evidence is required to substantiate numerous suggestions in the literature that climatic factors are related to, or cause, certain biological events. -

Database of Irish Lepidoptera. 1 - Macrohabitats, Microsites and Traits of Noctuidae and Butterflies
Database of Irish Lepidoptera. 1 - Macrohabitats, microsites and traits of Noctuidae and butterflies Irish Wildlife Manuals No. 35 Database of Irish Lepidoptera. 1 - Macrohabitats, microsites and traits of Noctuidae and butterflies Ken G.M. Bond and Tom Gittings Department of Zoology, Ecology and Plant Science University College Cork Citation: Bond, K.G.M. and Gittings, T. (2008) Database of Irish Lepidoptera. 1 - Macrohabitats, microsites and traits of Noctuidae and butterflies. Irish Wildlife Manual s, No. 35. National Parks and Wildlife Service, Department of the Environment, Heritage and Local Government, Dublin, Ireland. Cover photo: Merveille du Jour ( Dichonia aprilina ) © Veronica French Irish Wildlife Manuals Series Editors: F. Marnell & N. Kingston © National Parks and Wildlife Service 2008 ISSN 1393 – 6670 Database of Irish Lepidoptera ____________________________ CONTENTS CONTENTS ........................................................................................................................................................1 ACKNOWLEDGEMENTS ....................................................................................................................................1 INTRODUCTION ................................................................................................................................................2 The concept of the database.....................................................................................................................2 The structure of the database...................................................................................................................2 -
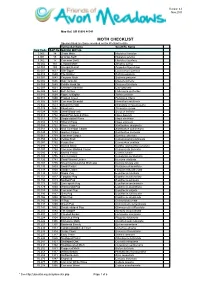
MOTH CHECKLIST Species Listed Are Those Recorded on the Wetland to Date
Version 4.0 Nov 2015 Map Ref: SO 95086 46541 MOTH CHECKLIST Species listed are those recorded on the Wetland to date. Vernacular Name Scientific Name New Code B&F No. MACRO MOTHS 3.005 14 Ghost Moth Hepialus humulae 3.001 15 Orange Swift Hepialus sylvina 3.002 17 Common Swift Hepialus lupulinus 50.002 161 Leopard Moth Zeuzera pyrina 54.008 169 Six-spot Burnet Zygaeba filipendulae 66.007 1637 Oak Eggar Lasiocampa quercus 66.010 1640 The Drinker Euthrix potatoria 68.001 1643 Emperor Moth Saturnia pavonia 65.002 1646 Oak Hook-tip Drepana binaria 65.005 1648 Pebble Hook-tip Drepana falcataria 65.007 1651 Chinese Character Cilix glaucata 65.009 1653 Buff Arches Habrosyne pyritoides 65.010 1654 Figure of Eighty Tethia ocularis 65.015 1660 Frosted Green Polyploca ridens 70.305 1669 Common Emerald Hermithea aestivaria 70.302 1673 Small Emerald Hemistola chrysoprasaria 70.029 1682 Blood-vein Timandra comae 70.024 1690 Small Blood-vein Scopula imitaria 70.013 1702 Small Fan-footed Wave Idaea biselata 70.011 1708 Single-dotted Wave Idaea dimidiata 70.016 1713 Riband Wave Idaea aversata 70.053 1722 Flame Carpet Xanthorhoe designata 70.051 1724 Red Twin-spot Carpet Xanthorhoe spadicearia 70.049 1728 Garden Carpet Xanthorhoe fluctuata 70.061 1738 Common Carpet Epirrhoe alternata 70.059 1742 Yellow Shell Camptogramma bilineata 70.087 1752 Purple Bar Cosmorhoe ocellata 70.093 1758 Barred Straw Eulithis (Gandaritis) pyraliata 70.097 1764 Common Marbled Carpet Chloroclysta truncata 70.085 1765 Barred Yellow Cidaria fulvata 70.100 1776 Green Carpet Colostygia pectinataria 70.126 1781 Small Waved Umber Horisme vitalbata 70.107 1795 November/Autumnal Moth agg Epirrita dilutata agg.