Arginine in the Critically
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CCA One Care Options Formulary
Commonwealth Care Alliance One Care Plan (Medicare-Medicaid Plan) 2021 List of Covered Drugs (Formulary) 30 Winter Street • Boston, MA 02108 PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN For more recent information or other questions, contact Commonwealth Care Alliance Member Services at 1-866-610-2273 (TTY: call MassRelay at 711), 8 a.m. – 8 p.m., 7 days a week, or visit www.commonwealthonecare.org H0137_CF2021 Approved Formulary: ID 00021588 • Version 13 • Updated on 08/01/2021 One Care Plan | 2021 List of Covered Drugs (Formulary) Introduction This document is called the List of Covered Drugs (also known as the Drug List). It tells you which prescription drugs, over-the-counter drugs and items are covered by Commonwealth Care Alliance. The Drug List also tells you if there are any special rules or restrictions on any drugs covered by One Care. Key terms and their definitions appear in the last chapter of the Member Handbook. Table of Contents A. Disclaimers ........................................................................................................................ 4 B. Frequently Asked Questions (FAQ) .................................................................................. 5 What prescription drugs are on the List of Covered Drugs? (We call the List of Covered Drugs the “Drug List” for short.) ................................................................... 5 B2. Does the Drug List ever change? ............................................................................... 5 B3. What happens when there is a change to the Drug List? ........................................... 6 B4. Are there any restrictions or limits on drug coverage or any required actions to take to get certain drugs? .................................................................................................. 7 B5. How will you know if the drug you want has limitations or if there are required actions to take to get the drug? ................................................................................. -

List of Toxic Chemicals Within the Glycol Ethers Category
United States Office of Environmental Revised December 2000 Environmental Protection Information EPA 745-R-00-004 Agency Washington, DC 20460 TOXICS RELEASE INVENTORY List of Toxic Chemicals within the Glycol Ethers Category Section 313 of the Emergency Planning and Community Right-to-Know Act (EPCRA) requires certain facilities manufacturing, processing, or otherwise using listed toxic chemicals to report their environmental releases of such chemicals annually. Beginning with the 1991 reporting year, such facilities also must report pollution prevention and recycling data for such chemicals, pursuant to section 6607 of the Pollution Prevention Act, 42 U.S.C. 13106. When enacted, EPCRA section 313 established an initial list of toxic chemicals that was comprised of more than 300 chemicals and 20 chemical categories. EPCRA section 313(d) authorizes EPA to add chemicals to or delete chemicals from the list, and sets forth criteria for these actions. CONTENTS Section 1. Introduction ...................................................... 3 Section 2. CAS Number List of Some Chemicals within the Glycol Ethers Category ........ 6 Section 3. CAS Number List of Some Mixtures That Contain Glycol Ethers within the Category .............................................. 185 Section 4. CAS Number List of Some Oligomeric or Polymeric Chemicals That Might Contain Glycol Ether Components within the Category .......................... 187 FOREWORD This document is an updated version of the previous document, EPA 745-R-99-006, June 1999. This version has the following updates: • The titles to Table 1 on page 6, Table 2 on page 185, and Table 3 on 187 are modified; and • The CAS number of second listing in Table 3 (Poly(oxy-1,2-ethanediyl), .alpha.- (phenylsulfonyl)-.omega.-methoxy-) on page 187 is changed from 7664-41-7 to 67584-43-4. -
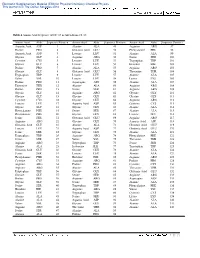
Table 2 Amino Acid Sequence of OC-17 As Taken from Ref. 28 Amino
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics This journal is © The Owner Societies 2012 Table 2 Amino Acid Sequence of OC-17 as taken from ref. 28 Amino Acid Abbr. Sequence Position Amino Acid Abbr. Sequence Position Amino Acid Abbr. Sequence Position Aspartic Acid ASP 1 Alanine ALA 49 Arginine ARG 97 Proline PRO 2 Glutamic Acid GLU 50 Phenyalanine PHE 98 Aspartic Acid ASP 3 Leucine LEU 51 Alanine ALA 99 Glycine GLY 4 Arginine ARG 52 Serine SER 100 Cysteine CYS 5 Leucine LEU 53 Tryptophan TRP 101 Glycine GLY 6 Leucine LEU 54 Histidine HIE 102 Proline PRO 7 Alanine ALA 55 Arginine ARG 103 Glycine GLY 8 Glutamic Acid GLU 56 Threonine THR 104 Tryptophan TRP 9 Leucine LEU 57 Alanine ALA 105 Valine VAL 10 Leucine LEU 58 Lysine LYS 106 Proline PRO 11 Asparagine ASN 59 Alanine ALA 107 Threonine THR 12 Alanine ALA 60 Arginine ARG 108 Proline PRO 13 Serine SER 61 Arginine ARG 109 Glycine GLY 14 Arginine ARG 62 Glycine GLY 110 Glycine GLY 15 Glycine GLY 63 Glycine GLY 111 Cysteine CYS 16 Glycine GLY 64 Arginine ARG 112 Leucine LEU 17 Aspartic Acid ASP 65 Cysteine CYS 113 Glycine GLY 18 Glycine GLY 66 Alanine ALA 114 Phenyalanine PHE 19 Serine SER 67 Alanine ALA 115 Phenyalanine PHE 20 Glycine GLY 68 Leucine LEU 116 Serine SER 21 Glutamic Acid GLU 69 Arginine ARG 117 Arginine ARG 22 Glycine GLY 70 Aspartic Acid ASP 118 Glutamic Acid GLU 23 Alanine ALA 71 Glutamic Acid GLU 119 Leucine LEU 24 Aspartic Acid ASP 72 Glutamic Acid GLU 120 Serine SER 25 Glycine GLY 73 Alanine ALA 121 Tryptophan TRP 26 Arginine ARG 74 Phenyalanine -

The Fate of Arginine and Proline Carbon in Squid Tissuesl
Pacific Science (1982), vol. 36, no. 3 © 1983 by the University of Hawaii Press. All rights reserved The Fate of Arginine and Proline Carbon in Squid Tissuesl T. P. MOMMSEN,2 C. J. FRENCH,2 B. EMMETI,2 and P. W. HOCHACHKA2 ABSTRACT: The metabolism of proline and arginine was investigated in kidney, gill, and heart of the pelagic squid, Symplectoteuthis. The rates of CO2 release from 14C-proline exceeded the rates from 14C-arginine. The metabolic rate of arginine and proline was assessed by monitoring the incorporation of arginine-derived carbon into various intermediates. Arginine was metabolized, through ornithine, to proline as well as to glutamate and various subsequent derivatives (alanine, octopine, aspartate, and carboxylic acids). The same com ponents became labeled using 14C-proline as the starting substrate, but only the gill was capable ofconverting proline to arginine via the urea cycle. In addition, 14C-proline oxidation rates were high enough to exceed those of 14C-glucose in at least three tissues, kidney, heart, and inner mantle muscle. AT LEAST IN PART because ofthe large pool size data for heart, gill, and kidney from the squid, of free amino acids in cephalopod muscles Symplectoteuthis, showing the capacity for ar (e.g., see Hochachka, French, and Meredith ginine conversion to proline. The conversion 1978), interest recently has been focusing ofproline to arginine was measurable only in on their possible roles in energy metabolism. the gill. Although qualitatively similar to re During metabolic studies on the 1979 Alpha sults obtained with other species, these data Helix Cephalopod Expedition, relatively high also show some important, tissue-specific dif rates of CO2 release from arginine and ferences (Mommsen et al. -

Arginine Is Synthesized from Proline, Not Glutamate, in Enterally Fed Human Preterm Neonates
0031-3998/11/6901-0046 Vol. 69, No. 1, 2011 PEDIATRIC RESEARCH Printed in U.S.A. Copyright © 2010 International Pediatric Research Foundation, Inc. Arginine Is Synthesized From Proline, Not Glutamate, in Enterally Fed Human Preterm Neonates CHRIS TOMLINSON, MAHROUKH RAFII, MICHAEL SGRO, RONALD O. BALL, AND PAUL PENCHARZ Department of Paediatrics [C.T., M.S., P.P.], Research Institute [C.T., M.R., P.P.], The Hospital for Sick Children, Toronto, Ontario M5G1X8, Canada; Department of Nutritional Sciences [C.T., M.S., P.P.], University of Toronto, Toronto, Ontario M5S3E2, Canada; Department of Paediatrics [M.S.], St Michael’s Hospital, Toronto, Ontario M5B1W8, Canada; Department of Agricultural, Food and Nutritional Science [R.O.B., P.P.], University of Alberta, Edmonton, Alberta T6G2P5, Canada ABSTRACT: In neonatal mammals, arginine is synthesized in the litis (NEC) (8) and pulmonary hypertension (9). Furthermore, enterocyte, with either proline or glutamate as the dietary precursor. arginine supplementation was shown to reduce the incidence We have shown several times in piglets that proline is the only of all stages of NEC in moderately at risk infants (10) and a precursor to arginine, although in vitro evidence supports glutamate single bolus infusion of i.v. arginine improved oxygenation in in this role. Because of this uncertainty, we performed a multitracer infants with pulmonary hypertension (11). Therefore, because stable isotope study to determine whether proline, glutamate, or both are dietary precursors for arginine in enterally fed human neonates. arginine is clearly important for metabolism in the neonate, it Labeled arginine (M ϩ 2), proline (M ϩ 1), and glutamate (M ϩ 3) is critical to understand the metabolic pathways involved in its were given enterally to 15 stable, growing preterm infants (GA at synthesis. -

Amino Acid Transport Pathways in the Small Intestine of the Neonatal Rat
Pediat. Res. 6: 713-719 (1972) Amino acid neonate intestine transport, amino acid Amino Acid Transport Pathways in the Small Intestine of the Neonatal Rat J. F. FITZGERALD1431, S. REISER, AND P. A. CHRISTIANSEN Departments of Pediatrics, Medicine, and Biochemistry, and Gastrointestinal Research Laboratory, Indiana University School of Medicine and Veterans Administration Hospital, Indianapolis, Indiana, USA Extract The activity of amino acid transport pathways in the small intestine of the 2-day-old rat was investigated. Transport was determined by measuring the uptake of 1 mM con- centrations of various amino acids by intestinal segments after a 5- or 10-min incuba- tion and it was expressed as intracellular accumulation. The neutral amino acid transport pathway was well developed with intracellular accumulation values for leucine, isoleucine, valine, methionine, tryptophan, phenyl- alanine, tyrosine, and alanine ranging from 3.9-5.6 mM/5 min. The intracellular accumulation of the hydroxy-containing neutral amino acids threonine (essential) and serine (nonessential) were 2.7 mM/5 min, a value significantly lower than those of the other neutral amino acids. The accumulation of histidine was also well below the level for the other neutral amino acids (1.9 mM/5 min). The basic amino acid transport pathway was also operational with accumulation values for lysine, arginine and ornithine ranging from 1.7-2.0 mM/5 min. Accumulation of the essential amino acid lysine was not statistically different from that of nonessential ornithine. Ac- cumulation of aspartic and glutamic acid was only 0.24-0.28 mM/5 min indicating a very low activity of the acidic amino acid transport pathway. -

Increased Arginine Amino Aciduria/Urea Cycle Disorder
Newborn Screening ACT Sheet Increased Arginine Amino Aciduria/Urea Cycle Disorder Differential Diagnosis: Argininemia (ARG) Condition Description: The urea cycle is the enzyme cycle whereby ammonia is converted to urea. In argininemia, defects in arginase, a urea cycle enzyme, may result in hyperammonemia. Take the Following IMMEDIATE Actions • Contact family to inform them of the newborn screening result and ascertain clinical status (poor feeding, vomiting, lethargy, tachypnea). • Immediate telephone consultation with pediatric metabolic specialist. (See attached list.) • Evaluate the newborn (poor feeding, vomiting, lethargy, hypotonia, tachypnea, seizures and signs of liver disease). • If any sign is present or infant is ill, IMMEDIATELY initiate emergency treatment for hyperammonemia in consultation with metabolic specialist. • Transport to hospital for further treatment in consultation with metabolic specialist. • Initiate timely confirmatory/diagnostic testing and management, as recommended by specialist. • Initial testing: immediate plasma ammonia, plasma quantitative amino acids, and urine orotic acid. • Repeat newborn screen if second screen has not been done. • Provide family with basic information about hyperammonemia. • Report findings to newborn screening program. Diagnostic Evaluation: Specific diagnosis is made by plasma quantitative amino acid analysis revealing increased arginine and urine orotic acid analysis revealing increased orotic acid, respectively. Blood ammonia determination may also reveal hyperammonemia. Clinical -

TR-511: Dipropylene Glycol (CASRN 25265-71-8) in F344/N Rats And
NTP TECHNICAL REPORT ON THE TOXICOLOGY AND CARCINOGENESIS STUDIES OF DIPROPYLENE GLYCOL (CAS NO. 25265-71-8) IN F344/N RATS AND B6C3F1 MICE (DRINKING WATER STUDIES) NATIONAL TOXICOLOGY PROGRAM P.O. Box 12233 Research Triangle Park, NC 27709 June 2004 NTP TR 511 NIH Publication No. 04-4445 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health FOREWORD The National Toxicology Program (NTP) is made up of four charter agencies of the U.S. Department of Health and Human Services (DHHS): the National Cancer Institute (NCI), National Institutes of Health; the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health; the National Center for Toxicological Research (NCTR), Food and Drug Administration; and the National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. In July 1981, the Carcinogenesis Bioassay Testing Program, NCI, was transferred to the NIEHS. The NTP coordinates the relevant programs, staff, and resources from these Public Health Service agencies relating to basic and applied research and to biological assay development and validation. The NTP develops, evaluates, and disseminates scientific information about potentially toxic and hazardous chemicals. This knowledge is used for protecting the health of the American people and for the primary prevention of disease. The studies described in this Technical Report were performed under the direction of the NIEHS and were conducted in compliance with NTP laboratory health and safety requirements and must meet or exceed all applicable federal, state, and local health and safety regulations. Animal care and use were in accordance with the Public Health Service Policy on Humane Care and Use of Animals. -

The Physiological Effects of Amino Acids Arginine and Citrulline
beverages Review The Physiological Effects of Amino Acids Arginine and Citrulline: Is There a Basis for Development of a Beverage to Promote Endurance Performance? A Narrative Review of Orally Administered Supplements Hollie Speer 1 , Nathan M. D’Cunha 1 , Michael J. Davies 1,2, Andrew J. McKune 1,3 and Nenad Naumovski 1,* 1 Faculty of Health, University of Canberra, Canberra, ACT 2601, Australia; [email protected] (H.S.); [email protected] (N.M.D.); [email protected] (M.J.D.); [email protected] (A.J.M.) 2 University of Canberra Research Institute for Sport and Exercise (UCRISE), Canberra, ACT 2601, Australia 3 Discipline of Biokinetics, Exercise and Leisure Sciences, School of Health Sciences, University of KwaZulu-Natal, Durban, KwaZulu-Natal 4000, South Africa * Correspondence: [email protected]; Tel.: +61-2-6206-8719 Received: 23 December 2019; Accepted: 14 February 2020; Published: 21 February 2020 Abstract: Nutritional and ergogenic aid supplementation is prevalent within athletic or general fitness populations, and is only continuing to gain momentum. Taken in isolation or as a combination, amino acid (AA) supplementation has the potential to increase endurance performance among other benefits. L-Arginine (L-Arg) and L-Citrulline (L-Cit) are two AAs proposed to increase endothelial nitric oxide (NO) synthesis, with potential additional physiological benefits, and therefore may contribute to enhanced performance outcomes such as increased power output, or time to exhaustion. However, the appropriate dose for promoting physiological and performance benefits of these AAs, and their potential synergistic effects remains to be determined. -

Safety Assessment of Α-Amino Acids As Used in Cosmetics
Safety Assessment of α-Amino Acids as Used in Cosmetics Status: Final Report for Public Distribution Release Date: October 5, 2012 Panel Meeting Date: September 10-11, 2012 The 2012 Cosmetic Ingredient Review Expert Panel members are: Chairman, Wilma F. Bergfeld, M.D., F.A.C.P.; Donald V. Belsito, M.D.; Curtis D. Klaassen, Ph.D.; Daniel C. Liebler, Ph.D.; Ronald A. Hill, Ph.D. James G. Marks, Jr., M.D.; Ronald C. Shank, Ph.D.; Thomas J. Slaga, Ph.D.; and Paul W. Snyder, D.V.M., Ph.D. The CIR Director is F. Alan Andersen, Ph.D. This safety assessment was prepared by Christina L. Burnett, Scientific Analyst/Writer, and Bart Heldreth, Ph.D., Chemist CIR. © Cosmetic Ingredient Review 1101 17th Street, NW, Suite 412 Washington, DC 20036-4702 ph 202.331.0651 fax 202.331.0088 [email protected] 1 ABSTRACT The Cosmetic Ingredient Review Expert Panel (the Panel) reviewed the safety of α-amino acids, which function primarily as hair and skin conditioning agents in cosmetic products. The safety of α-amino acids as direct food additives has been well established based on extensive research through acute and chronic dietary exposures. The Panel focused its review on dermal irritation and sensitization data relevant to the use of these ingredients in topical cosmetics. The Panel concluded that α-amino acids were safe as cosmetic ingredients in the practices of use and concentration of this safety assessment. INTRODUCTION Amino acids and their salts are widely used as cosmetic ingredients, and function primarily as hair conditioning agents and skin conditioning agents (humectant and miscellaneous). -

Dietary Interrelationships Among Arginine, Methionine, and Lysine In
Downloaded from https://www.cambridge.org/core British Journal of Nutrition (2002), 88, 655–660 DOI: 10.1079/BJN2002732 q The Authors 2002 Dietary interrelationships among arginine, methionine, and lysine in . IP address: young broiler chicks 170.106.202.226 M. Chamruspollert, G. M. Pesti* and R. I. Bakalli Department of Poultry Science, The University of Georgia, Athens, Georgia 30602-2772, USA (Received 5 September 2001 – Revised 30 July 2002 – Accepted 11 August 2002) , on 26 Sep 2021 at 15:14:48 Since excess dietary lysine (Lys) can increase the chick’s arginine (Arg) requirement and excess Arg can increase the chick’s methionine (Met) requirement, experiments were conducted to test the hypothesis that responses to dietary Lys and Met are also interrelated. Day-old Ross £ Ross chicks were fed a maize–soyabean meal-based diet supplemented with four levels of , subject to the Cambridge Core terms of use, available at L-Arg (0, 5, 10 or 20 g/kg), factorially arranged with four levels of supplemental DL-Met (0, 1, 2 or 3 g/kg). Three replicate pens of ten chicks each were randomly assigned to each treatment and fed for 14 d. An increase in Arg in the diet caused growth and feed-intake depression (P¼0·0001), but increasing Met in the diet enhanced growth and feed intake (P¼0·0001). Arg toxicity was dependent on the Met level of the diet (Arg £ Met interaction; P¼0·0153). Experiment 2 was conducted to study interrelationships among Arg, Met, and Lys. Eight treatments were factorially combined: two levels of supplemental L-Arg (0 or 10 g/kg), two levels of supplemental DL-Met (0 or 2 g/kg), and two levels of supplemental L-Lys (0 or 6 g/kg). -

Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act
Updated June 07, 2021 Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act Three categories of bulk drug substances: • Category 1: Bulk Drug Substances Under Evaluation • Category 2: Bulk Drug Substances that Raise Significant Safety Risks • Category 3: Bulk Drug Substances Nominated Without Adequate Support Updates to Categories of Substances Nominated for the 503B Bulk Drug Substances List1 • Add the following entry to category 2 due to serious safety concerns of mutagenicity, cytotoxicity, and possible carcinogenicity when quinacrine hydrochloride is used for intrauterine administration for non- surgical female sterilization: 2,3 o Quinacrine Hydrochloride for intrauterine administration • Revision to category 1 for clarity: o Modify the entry for “Quinacrine Hydrochloride” to “Quinacrine Hydrochloride (except for intrauterine administration).” • Revision to category 1 to correct a substance name error: o Correct the error in the substance name “DHEA (dehydroepiandosterone)” to “DHEA (dehydroepiandrosterone).” 1 For the purposes of the substance names in the categories, hydrated forms of the substance are included in the scope of the substance name. 2 Quinacrine HCl was previously reviewed in 2016 as part of FDA’s consideration of this bulk drug substance for inclusion on the 503A Bulks List. As part of this review, the Division of Bone, Reproductive and Urologic Products (DBRUP), now the Division of Urology, Obstetrics and Gynecology (DUOG), evaluated the nomination of quinacrine for intrauterine administration for non-surgical female sterilization and recommended that quinacrine should not be included on the 503A Bulks List for this use. This recommendation was based on the lack of information on efficacy comparable to other available methods of female sterilization and serious safety concerns of mutagenicity, cytotoxicity and possible carcinogenicity in use of quinacrine for this indication and route of administration.