PRESS RELEASE Adocia Is Now Integrated Into the CAC Mid 60
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Case Study: the Bayer-Monsanto Acquisition Deal
Case Study: The Bayer-Monsanto Acquisition Deal Student: Georgios Ritsos-Kokkinis (SNR:2013707, ANR:927674) Supervisor: Prof. Dr. Christoph Schneider Second reader: Prof. Dr. Olivier de Jonghe Tilburg, August 2020 Acknowledgements I would like to thank my supervisor Dr. Christoph Schneider, Assistant Professor at Tilburg University – Department of Finance, whose expertise and guidance were invaluable in soundly formulating all the relevant methodologies, and concisely structuring the output results. Your feedback pushed me to sharpen my thinking and brought my work to a higher level. I would also like to thank fellow scholar Thanos Vasiliadis, PhD candidate in Mathematics at Université côté d'Azur, Laboratoire Dieudonné, for his support, encouragement and constructive comments, which were instrumental to the completion of this dissertation. ABSTRACT The acquisition of Bayer-Monsanto was one of the largest M&A deals of the past decade. In this study, I document the motives of this transaction and the timeline of events that took place, from the initial rumors until Bayer’s milestone settlement of Monsanto’s “Roundup” litigation in mid-2020. Fundamental valuations of the two individual companies and their officially disclosed synergies reveal that the premium paid over Monsanto’s overvalued share price was not justified, as is commonly observed in the M&A ecosystem. Multiple approaches to the calculation of the discount factor reach the same conclusion. Averaging across multiple costs of capital, shows that the combined intrinsic value with synergy of roughly $177bn was some $2.12bn higher than the sum of parts, suggesting a rather profitable investment opportunity. However, Bayer, did overpay by 18.08% to reap these benefits. -
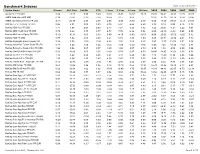
Benchmark Indexes Data As Of: 03/31/2011 Index Name Sharpe Std
Benchmark Indexes Data as of: 03/31/2011 Index Name Sharpe Std. Dev. 1st Qtr YTD 1 Year 3 Year 5 Year 10 Year 2010 2009 2008 2007 2006 Alerian MLP TR USD 0.89 23.25 5.99 5.99 33.02 20.03 16.57 17.25 35.85 76.41 -36.91 12.72 26.07 AMEX Gold Miners PR USD 0.39 47.82 -2.15 -2.15 35.65 8.12 9.68 - 33.94 37.30 -26.79 16.86 21.86 AMEX International Market PR USD -0.13 25.37 2.77 2.77 4.05 -5.95 -0.80 2.17 0.80 17.60 -39.51 16.18 21.51 BarCap 1-5 Yr Treasury TR USD 0.98 2.51 0.00 0.00 2.79 2.91 4.88 4.20 3.73 0.19 8.83 8.16 3.75 BarCap ABS Auto TR USD 1.17 5.68 0.63 0.63 2.47 7.21 6.23 5.12 3.25 26.07 -5.57 5.22 4.61 BarCap ABS Credit Card TR USD 0.79 9.42 0.77 0.77 4.73 7.76 6.86 5.84 6.55 29.89 -9.33 5.45 4.55 BarCap ABS Home Equity TR USD -0.19 16.91 0.00 0.00 0.00 -4.10 -5.80 -0.53 0.00 20.63 -36.00 -8.25 5.32 BarCap ABS TR USD 0.77 6.92 0.64 0.64 4.21 5.74 4.36 4.59 5.85 24.71 -12.72 2.21 4.70 BarCap Aggregate Bond Treasury TR 0.58 5.58 -0.16 -0.16 4.53 3.55 5.69 5.15 5.87 -3.57 13.74 9.01 3.08 BarCap Asian Pac Taiwan Dollar TR JPY 0.78 6.24 1.54 1.54 -2.45 -1.00 -2.50 -0.66 -2.26 7.45 -11.24 -7.52 2.81 BarCap Bellwether Swap 6 Mon TR USD 1.92 0.56 0.07 0.07 0.39 1.50 3.07 2.79 0.38 1.58 4.09 5.39 5.54 BarCap CMBS ERISA Eligible TR USD 0.53 18.46 2.05 2.05 12.62 8.79 6.87 6.53 20.40 28.45 -20.52 5.58 4.72 BarCap Credit 1-3 Yr TR USD 1.16 3.62 0.58 0.58 3.24 4.73 5.29 4.80 4.15 11.59 0.30 5.96 4.66 BarCap Credit 1-5 Yr TR USD 1.06 4.64 0.85 0.85 4.36 5.47 5.77 5.27 5.44 13.52 -1.13 6.09 4.69 BarCap Danish Krone Aggregate TR DKK 0.12 14.76 -

Press Release Paris – June 21St, 2021
Press Release Paris – June 21st, 2021 Europcar Mobility Group rejoins Euronext SBF 120 index Europcar Mobility Group, a major player in mobility markets, is pleased to announce that it has re-entered into the SBF 120 and CAC Mid 60 indices, in accordance with the decision taken by the Euronext Index Steering Committee. This re-entry, which took place after market close on Friday 18 June 2021, is effective from Monday 21 June 2021. The SBF 120 is one of the flagship indices of the Paris Stock Exchange. It includes the first 120 stocks listed on Euronext Paris in terms of liquidity and market capitalization. The CAC Mid 60 index includes 60 companies of national and European importance. It represents the 60 largest French equities beyond the CAC 40 and the CAC Next 20. It includes the 60 most liquid stocks listed in Paris among the 200 first French capitalizations. This total of 120 companies compose the SBF 120. Caroline Parot, CEO of Europcar Mobility Group, said: “Europcar Mobility Group welcomes the re-integration of the SBF 120 and CAC Mid 60. This follows the successful closing of the Group’s financial restructuring during Q1 2021, which allowed the Group to open a new chapter in its history. Europcar Mobility Group re-entry into the SBF 120 and CAC Mid 60 is an important step for our Group and recognizes the fact that we are clearly "back in the game”, in a good position to take full advantage of the progressive recovery of the Travel and Leisure market. In that perspective, our teams are fully mobilized for summer of 2021, in order to meet the demand and expectations of customers who are more than ever eager to travel”. -

Why Switzerland?
Why Switzerland? Marwan Naja, AS Investment Management January, 2010 I. Executive Summary Swiss Equities Have Outperformed: Here is a fact you probably did not know: The Swiss equity market has arguably been the best performing developed world market over the past 20, 10, 5 and 2 years. The SPI Index, which incorporates over 200 Swiss stocks, is the best performing index in Swiss Franc (“CHF”), US Dollars (“USD”), Euro (“EUR”) and British Pounds (“GBP”) over the past 20 years1,10 years, 5 years, and 2 years when compared to similar broad-based total return indices in the United States (S&P 500 Total Return), the United Kingdom (FTSE 350 Total Return), Germany (CDAX), France (SBF 120 Total Return) and Japan (TOPIX Total Return)2. For most of these periods the magnitude of the outperformance is significant. Furthermore, the Swiss market has exhibited attractive risk characteristics including lower volatility than comparable markets. Figure 1: SPI 20 Year Performance (red) Compared to Major Developed Indices in CHF3 1 The 20 year comparable excludes the EUR which has not existed for that duration and the French SBF 120 TR which was established in 1990 and has underperformed the SPI over the 19 year period. 2 The Swiss market is the best performer in our local currency comparison (stripping out the foreign exchange effects) over 20 years and marginally trails the FTSE 350 for the 10 year, 5 year and 2 year comparisons. 3 Source of all graphs is Bloomberg unless otherwise indicated. Why Switzerland? AS Investment Management Contents I. Executive Summary ............................................................................................................ 1 Swiss Equities Have Outperformed ................................................................................... -

Eurofins Fact Sheet March 2021
FACT SHEET Company Overview Our Vision: To be the Global Leader in Eurofins is Testing for Life. Eurofins is the global leader in food, environment, pharmaceutical and Testing for Life cosmetic product testing and in agroscience Contract Research services. Eurofins is also one of the market leaders in certain testing and laboratory services for genomics, discovery pharmacology, Our Mission: To contribute to a safer and forensics, advanced material sciences and in the support of clinical studies, as well as having an healthier world by providing our customers emerging global presence as a Contract Development and Manufacturing Organisation. The Group with innovative and high quality laboratory, also has a rapidly developing presence in highly specialised and molecular clinical diagnostic testing research and advisory services, whilst and in-vitro diagnostic products. creating opportunities for our employees and generating sustainable shareholder value History Eurofins Scientific was founded in 1987 with four employees to market the SNIF-NMR technology, Our Values: Customer Focus, Quality, a patented analytical method used to verify the origin and purity of several types of food and Competence & Team Spirit and Integrity beverages and identify sophisticated fraud not detectable by other methods. Today, the Eurofins Group is a leading provider of testing and analytical services with: • an international network of more than 800 laboratories in over 50 countries Revenues in EUR million • over 50,000 staff • a portfolio of over 200,000 analytical methods • more than 450 million tests performed each year Eurofins has been one of the fastest growing listed European companies. Since its IPO on the French stock exchange in 1997, Eurofins’ revenues have increased by 34% each year (in compound average) to over EUR 5.4 billion in 2020. -

Financial Market Data for R/Rmetrics
Financial Market Data for R/Rmetrics Diethelm Würtz Andrew Ellis Yohan Chalabi Rmetrics Association & Finance Online R/Rmetrics eBook Series R/Rmetrics eBooks is a series of electronic books and user guides aimed at students and practitioner who use R/Rmetrics to analyze financial markets. A Discussion of Time Series Objects for R in Finance (2009) Diethelm Würtz, Yohan Chalabi, Andrew Ellis R/Rmetrics Meielisalp 2009 Proceedings of the Meielisalp Workshop 2011 Editor Diethelm Würtz Basic R for Finance (2010), Diethelm Würtz, Yohan Chalabi, Longhow Lam, Andrew Ellis Chronological Objects with Rmetrics (2010), Diethelm Würtz, Yohan Chalabi, Andrew Ellis Portfolio Optimization with R/Rmetrics (2010), Diethelm Würtz, William Chen, Yohan Chalabi, Andrew Ellis Financial Market Data for R/Rmetrics (2010) Diethelm W?rtz, Andrew Ellis, Yohan Chalabi Indian Financial Market Data for R/Rmetrics (2010) Diethelm Würtz, Mahendra Mehta, Andrew Ellis, Yohan Chalabi Asian Option Pricing with R/Rmetrics (2010) Diethelm Würtz R/Rmetrics Singapore 2010 Proceedings of the Singapore Workshop 2010 Editors Diethelm Würtz, Mahendra Mehta, David Scott, Juri Hinz R/Rmetrics Meielisalp 2011 Proceedings of the Meielisalp Summer School and Workshop 2011 Editor Diethelm Würtz III tinn-R Editor (2010) José Cláudio Faria, Philippe Grosjean, Enio Galinkin Jelihovschi and Ri- cardo Pietrobon R/Rmetrics Meielisalp 2011 Proceedings of the Meielisalp Summer Scholl and Workshop 2011 Editor Diethelm Würtz R/Rmetrics Meielisalp 2012 Proceedings of the Meielisalp Summer Scholl and Workshop 2012 Editor Diethelm Würtz Topics in Empirical Finance with R and Rmetrics (2013), Patrick Hénaff FINANCIAL MARKET DATA FOR R/RMETRICS DIETHELM WÜRTZ ANDREW ELLIS YOHAN CHALABI RMETRICS ASSOCIATION &FINANCE ONLINE Series Editors: Prof. -

Neoen Joins the SBF 120Index
Paris, June 11, 2020 Neoen joins the SBF 120 index Neoen (ISIN: FR0011675362, Ticker: NEOEN), one of the world’s leading and fastest-growing independent producers of exclusively renewable energy, is announcing it is joining the SBF 120 index consisting of the top 120 stocks listed on the Euronext Paris market in term of both liquidity and market capitalization. As part of its quarterly review of the Euronext Paris indices, the Index Committee decided to include Neoen in both the SBF 120 and the CAC Mid 60 indices. This decision will take effect from June 22, 2020. Neoen’s inclusion in these indices is a testament to its good share price performance since its October 2018 IPO. Over the past 20 months, the Company’s market capitalization has more than doubled to almost €3 billion. This strong rise was accompanied by a steady increase in trading volumes. Since its IPO, Neoen has consistently delivered strong earnings growth and expansion in its project portfolio. Assets in operation or under construction have increased from 1.8 GW at June 30, 2018 to 3.1 GW at March 31, 2020, and the target is to have more than 5 GW by year-end 2021. This rapid and profitable growth has been founded on geographical and technological diversification of its asset base. Neoen is present in 14 countries on 4 continents and it develops and operates solar and wind energy generation and storage capacity. Xavier Barbaro, Neoen’s Chairman and Chief Executive Officer, commented: “We are very pleased to be joining this benchmark index, which marks a new important step for Neoen. -

Final Report Amending ITS on Main Indices and Recognised Exchanges
Final Report Amendment to Commission Implementing Regulation (EU) 2016/1646 11 December 2019 | ESMA70-156-1535 Table of Contents 1 Executive Summary ....................................................................................................... 4 2 Introduction .................................................................................................................... 5 3 Main indices ................................................................................................................... 6 3.1 General approach ................................................................................................... 6 3.2 Analysis ................................................................................................................... 7 3.3 Conclusions............................................................................................................. 8 4 Recognised exchanges .................................................................................................. 9 4.1 General approach ................................................................................................... 9 4.2 Conclusions............................................................................................................. 9 4.2.1 Treatment of third-country exchanges .............................................................. 9 4.2.2 Impact of Brexit ...............................................................................................10 5 Annexes ........................................................................................................................12 -

Trends in the European Audit Market
Audit Analytics® Trends in the European Audit Market MAY 2019 A Review of the Audit Environment in Europe TableAbout Audit Analytics of .................................................................................................................................................................................Contents 1 Audit Analytics Europe ......................................................................................................................................................................... 1 Our Value .................................................................................................................................................................................................... 1 Our Mission ................................................................................................................................................................................................ 1 Data Collection ......................................................................................................................................................................................... 2 About this Report ......................................................................................................................................................................................... 3 Introduction .................................................................................................................................................................................................... 3 Market -

Execution Version GUARANTEED SENIOR SECURED NOTES
Execution Version GUARANTEED SENIOR SECURED NOTES PROGRAMME issued by GOLDMAN SACHS INTERNATIONAL in respect of which the payment and delivery obligations are guaranteed by THE GOLDMAN SACHS GROUP, INC. (the “PROGRAMME”) PRICING SUPPLEMENT DATED 21 DECEMBER 2020 SERIES 2020-20 SENIOR SECURED FIXED RATE NOTES (the “SERIES”) ISIN: XS2277589615 Common Code: 227758961 This document constitutes the Pricing Supplement of the above Series of Secured Notes (the “Secured Notes”) and must be read in conjunction with the Base Listing Particulars dated 25 September 2020, as supplemented and amended by Prospectus Supplement No. 1 dated 20 October 2020, Prospectus Supplement No. 2 dated 2 November 2020 and Prospectus Supplement No. 3 dated 12 November 2020 (as so amended the “Base Listing Particulars”), and in particular, the Base Terms and Conditions of the Secured Notes, as set out therein. Full information on the Issuer, The Goldman Sachs Group. Inc. (the “Guarantor”), and the terms and conditions of the Secured Notes, is only available on the basis of the combination of this Pricing Supplement and the Base Listing Particulars as so supplemented. The Base Listing Particulars has been published at www.ise.ie and is available for viewing during normal business hours at the registered office of the Issuer, and copies may be obtained from the specified office of the listing agent in Ireland. The Issuer accepts responsibility for the information contained in this Pricing Supplement. To the best of the knowledge and belief of the Issuer and the Guarantor the information contained in the Base Listing Particulars, as completed by this Pricing Supplement in relation to the Series of Secured Notes referred to above, is true and accurate in all material respects and, in the context of the issue of this Series, there are no other material facts the omission of which would make any statement in such information misleading. -

Execution Version
Execution Version GUARANTEED SENIOR SECURED NOTES PROGRAMME issued by GOLDMAN SACHS INTERNATIONAL in respect of which the payment and delivery obligations are guaranteed by THE GOLDMAN SACHS GROUP, INC. (the “PROGRAMME”) PRICING SUPPLEMENT DATED 23rd SEPTEMBER 2020 SERIES 2020-12 SENIOR SECURED EXTENDIBLE FIXED RATE NOTES (the “SERIES”) ISIN: XS2233188510 Common Code: 223318851 This document constitutes the Pricing Supplement of the above Series of Secured Notes (the “Secured Notes”) and must be read in conjunction with the Base Listing Particulars dated 25 September 2019, as supplemented from time to time (the “Base Prospectus”), and in particular, the Base Terms and Conditions of the Secured Notes, as set out therein. Full information on the Issuer, The Goldman Sachs Group. Inc. (the “Guarantor”), and the terms and conditions of the Secured Notes, is only available on the basis of the combination of this Pricing Supplement and the Base Listing Particulars as so supplemented. The Base Listing Particulars has been published at www.ise.ie and is available for viewing during normal business hours at the registered office of the Issuer, and copies may be obtained from the specified office of the listing agent in Ireland. The Issuer accepts responsibility for the information contained in this Pricing Supplement. To the best of the knowledge and belief of the Issuer and the Guarantor the information contained in the Base Listing Particulars, as completed by this Pricing Supplement in relation to the Series of Secured Notes referred to above, is true and accurate in all material respects and, in the context of the issue of this Series, there are no other material facts the omission of which would make any statement in such information misleading. -

ANNEX Stock Indices Meeting the Requirements of Article 344 Of
DRAFT ITS ON DIVERSIFIED INDICES UNDER ARTICLE 344(4) OF REGULATION (EU) 575/2013 ANNEX Stock indices meeting the requirements of Article 344 of Regulation (EU) No 575/2013 Index Country\Area 1. STOXX Asia/Pacific 600 Asia/Pacific 2. ASX100 Australia 3. ASX200 Australia 4. S&P All Ords Australia 5. ATX Austria 6. ATX Prime Austria 7. BEL20 Belgium 8. SaoPaulo - Bovespa Brazil 9. TSX60 Canada 10. CETOP20 Index Central Europe 11. CSI 100 Index China 12. CSI 300 Index China 13. FTSE China A50 Index China 14. Hang Seng Mainland 100 China China 15. PX Global Prague Czech Republic 16. OMX Copenhagen 20 CAP Denmark 17. OMX Copenhagen 25 Denmark 18. OMX Copenhagen Benchmark Denmark 19. FTSE RAFI Developed 1000 Developed Markets 20. CECE Composite Index EUR Eastern Europe 21. FTSE RAFI Emerging Markets Emerging Markets 22. MSCI Emerging Markets 50 Emerging Markets 23. Bloomberg European 500 Europe 24. DJ Euro STOXX 50 Europe 25. FTSE Euro 100 Europe 26. FTSE Eurofirst 100 Europe 27. FTSE Eurofirst 300 Europe 28. FTSE Eurofirst 80 Europe 29. FTSE EuroMid Europe 30. FTSE Eurotop 100 Europe 31. MSCI Euro Europe 32. MSCI Europe Europe 33. MSCI Pan-Euro Europe 34. NTX New Europe Blue Chip Europe 35. S&P Euro Europe 36. S&P Europe 350 Europe 1 DRAFT ITS ON DIVERSIFIED INDICES UNDER ARTICLE 344(4) OF REGULATION (EU) 575/2013 37. STOXX All Europe 100 Europe 38. STOXX All Europe 800 Europe 39. STOXX Europe 50 Europe 40. STOXX Europe 600 Europe 41. STOXX Europe 600 Equal Weight Europe 42.