Novel Insights Into the Immunotherapy of Soft Tissue Sarcomas: Do We Need a Change of Perspective?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Study Protocol
Clovis Oncology, Inc. Clinical Protocol Oral rucaparib (CO-338) CO-338-014 7 July 2016 CONFIDENTIAL A Multicenter, Randomized, Double-Blind, Placebo-Controlled Phase 3 Study of Rucaparib as Switch Maintenance Following Platinum-Based Chemotherapy in Patients with Platinum-Sensitive, High-Grade Serous or Endometrioid Epithelial Ovarian, Primary Peritoneal or Fallopian Tube Cancer Protocol Number: CO-338-014 Investigational Product: Rucaparib (CO-338) Eudra CT Number: IND Number: Development Phase: Phase 3 Indications Studied: Platinum-sensitive, high-grade serous and endometrioid epithelial ovarian, primary peritoneal, and fallopian tube cancer Sponsor Name and Address: Clovis Oncology, Inc. 5500 Flatiron Parkway Suite 100 Boulder, CO 80301 USA Phone Number: 303-625-5000 Facsimile Number: 303-245-0360 Responsible Medical Officer: Compliance Statement: This study will be conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki, clinical research guidelines established by the Code of Federal Regulations (Title 21, CFR Parts 50, 56, and 312), and ICH GCP Guidelines. Essential study documents will be archived in accordance with applicable regulations. CONFIDENTIALITY STATEMENT The information in this document contains commercial information and trade secrets that are privileged or confidential and may not be disclosed unless such disclosure is required by applicable laws and regulations. In any event, persons to whom the information is disclosed must be informed that the information is privileged or confidential and may not be further disclosed by them. These restrictions on disclosure will apply equally to all future information supplied to you which is indicated as privileged or confidential. 1 Clovis Oncology, Inc. Clinical Protocol Oral rucaparib (CO-338) CO-338-014 Coordinating Investigators for the Study Coordinating Investigator for North America: Coordinating Investigator for Europe, Middle East, and Asia Pacific: 2 Clovis Oncology, Inc. -

Multi-Discipline Review/Summary, Clinical, Non-Clinical
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 209115Orig1s000 MULTI-DISCIPLINE REVIEW Summary / Clinical / Non-Clinical NDA/BLA Multi-disciplinary Review and Evaluation NDA 209115 Rubraca (rucaparib) 1 NDA/BLA Multi-disciplinary Review and Evaluation Application Type NDA Application Number(s) 209115 Priority or Standard Priority Submit Date(s) June 23, 2016 Received Date(s) June 23, 2016 PDUFA Goal Date February 23, 2017 Division/Office DOP1/OHOP Review Completion Date November 22, 2016 Established Name Rucaparib (Proposed) Trade Name RUBRACA Pharmacologic Class Poly (ADP-Ribose) Polymerase 1 inhibitor Code name AG-014669, PF-01367338, Applicant Clovis Oncology, Inc. Formulation(s) Oral tablet Dosing Regimen 600 mg PO BID Applicant Proposed Rubraca is indicated as monotherapy treatment of advanced ovarian Indication(s)/Population(s) cancer in patients with deleterious BRCA-mutated tumors, inclusive of both germline BRCA and somatic BRCA mutations (as detected by an FDA approved test), and who have been treated with two or more chemotherapies. Recommendation on Accelerated approval Regulatory Action Recommended Rubraca™ is indicated as monotherapy for the treatment of patients Indication(s)/Population(s) with deleterious BRCA mutation (germline and/or somatic) associated (if applicable) advanced ovarian cancer who have been treated with two or more chemotherapies. Select patients for therapy based on an FDA-approved companion diagnostic for Rubraca. 1-1 Version date: February 1, 2016 for initial rollout (NME/original BLA reviews) Reference ID: 40178304028244 NDA/BLA Multi-disciplinary Review and Evaluation NDA 209115 Rubraca (rucaparib) Contents 1 NDA/BLA Multi-disciplinary Review and Evaluation .......................................................................... 1-1 Reviewers of Multi-Disciplinary Review and Evaluation ........................................................................... -

Predictive QSAR Tools to Aid in Early Process Development of Monoclonal Antibodies
Predictive QSAR tools to aid in early process development of monoclonal antibodies John Micael Andreas Karlberg Published work submitted to Newcastle University for the degree of Doctor of Philosophy in the School of Engineering November 2019 Abstract Monoclonal antibodies (mAbs) have become one of the fastest growing markets for diagnostic and therapeutic treatments over the last 30 years with a global sales revenue around $89 billion reported in 2017. A popular framework widely used in pharmaceutical industries for designing manufacturing processes for mAbs is Quality by Design (QbD) due to providing a structured and systematic approach in investigation and screening process parameters that might influence the product quality. However, due to the large number of product quality attributes (CQAs) and process parameters that exist in an mAb process platform, extensive investigation is needed to characterise their impact on the product quality which makes the process development costly and time consuming. There is thus an urgent need for methods and tools that can be used for early risk-based selection of critical product properties and process factors to reduce the number of potential factors that have to be investigated, thereby aiding in speeding up the process development and reduce costs. In this study, a framework for predictive model development based on Quantitative Structure- Activity Relationship (QSAR) modelling was developed to link structural features and properties of mAbs to Hydrophobic Interaction Chromatography (HIC) retention times and expressed mAb yield from HEK cells. Model development was based on a structured approach for incremental model refinement and evaluation that aided in increasing model performance until becoming acceptable in accordance to the OECD guidelines for QSAR models. -

Development of Red Blood Cell Autoantibodies Following Treatment with Checkpoint Inhibitors
CASE R EP O RT Development of red blood cell autoantibodies following treatment with checkpoint inhibitors: a new class of anti-neoplastic, immunotherapeutic agents associated with immune dysregulation L.L.W. Cooling, J. Sherbeck, J.C. Mowers, and S.L. Hugan Ipilimumab, nivolumab, and pembrolizumab represent a new Table 1. Checkpoint inhibitors class of immunotherapeutic drugs for treating patients with Drug class (trade name, manufacturer) advanced cancer. Known as checkpoint inhibitors, these drugs act to upregulate the cellular and humoral immune response Anti-CTLA-4 to tumor antigens by inhibiting T-cell autoregulation. As a Ipilimumab (Yervoy, Bristol-Myers Squibb) consequence, they can be associated with immune-related adverse Tremelimumab (AstraZeneca, compassionate use only) events (irAEs) due to loss of self-tolerance, including rare cases of immune-related cytopenias. We performed a retrospective Anti-PD-1 clinical chart review, including serologic, hematology, and Nivolumab (Opdivo, Bristol-Myers Squibb) chemistry laboratory results, of two patients who developed Pembrolizumab (Keytruda, Merck Sharp & Dohme) red blood cell (RBC) autoantibodies during treatment with a Pidilizumab (Medivations, in clinical trials) checkpoint inhibitor. Serologic testing of blood samples from these patients during induction therapy with ipilimumab and Anti-PD-L1 nivolumab, respectively, showed their RBCs to be positive by Atezolizumab (Genentech, in clinical trials) the direct antiglobulin test (IgG+, C3+) and their plasma to Durvalumab (AstraZeneca, approved bladder cancer) contain panreactive RBC autoantibodies. Neither patient had evidence of hemolysis. Both patients developed an additional CTLA-4 = cytotoxic T-lymphocyte–associated antigen 4; PD-1 = programmed cell death protein 1; PD-L1 = programmed cell death ligand 1. -

Withdrawal Assessment Report - Orphan Maintenance
17 January 2019 EMA/22653/2019 Committee for Orphan Medicinal Products Withdrawal Assessment Report - Orphan Maintenance Rubraca (rucaparib) Treatment of ovarian cancer EU/3/12/1049 (EMA/OD/085/12) Sponsor: Clovis Oncology UK Limited Note Assessment report as adopted by the COMP with all information of a commercially confidential nature deleted. 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2019. Reproduction is authorised provided the source is acknowledged Table of contents 1. Product and administrative information .................................................. 3 2. Grounds for the COMP opinion at the designation stage .......................... 5 3. Review of criteria for orphan designation at the time of type II variation .................................................................................................................... 5 Article 3(1)(a) of Regulation (EC) No 141/2000 .............................................................. 5 Article 3(1)(b) of Regulation (EC) No 141/2000 .............................................................. 6 4. COMP list of issues .................................................................................. 8 Withdrawal Assessment Report - Orphan Maintenance EMA/22653/2019 Page 2/8 1. Product and administrative information Product Active substance Rucaparib International Non-Proprietary -

Systemic Immunotherapy for Urothelial Cancer: Current Trends and Future Directions
Review Systemic Immunotherapy for Urothelial Cancer: Current Trends and Future Directions Shilpa Gupta 1,†, David Gill 2,†, Austin Poole 2 and Neeraj Agarwal 2,* 1 Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA; [email protected] 2 Huntsman Cancer Institute, University of Utah, Salt Lake City, UT 84112, USA; [email protected] (D.G.); [email protected] (A.P.) * Correspondence: [email protected]; Tel.: +1-801-213-5658; Fax: +1-801-585-0124 † These authors contributed equally to this work. Academic Editor: Vita Golubovskaya Received: 14 December 2016; Accepted: 18 January 2017; Published: 27 January 2017 Abstract: Urothelial cancer of the bladder, renal pelvis, ureter, and other urinary organs is the fifth most common cancer in the United States, and systemic platinum-based chemotherapy remains the standard of care for first-line treatment of advanced/metastatic urothelial carcinoma (UC). Until recently, there were very limited options for patients who are refractory to chemotherapy, or do not tolerate chemotherapy due to toxicities and overall outcomes have remained very poor. While the role of immunotherapy was first established in non-muscle invasive bladder cancer in the 1970s, no systemic immunotherapy was approved for advanced disease until the recent approval of a programmed death ligand-1 (PD-L1) inhibitor, atezolizumab, in patients with advanced/metastatic UC who have progressed on platinum-containing regimens. This represents a significant milestone in this disease after a void of over 30 years. In addition to atezolizumab, a variety of checkpoint inhibitors have shown a significant activity in advanced/metastatic urothelial carcinoma and are expected to gain Food and Drug Administration (FDA) approval in the near future. -

ARIEL4: an International, Randomised Phase 3 Study Of
ARIEL4: An International, Randomised Phase 3 Study of Rucaparib vs Chemotherapy in BRCA1- or BRCA2-Mutated, Abstract ESGO7-0665 Relapsed Ovarian Cancer (OC) Rebecca S. Kristeleit,1 Domenica Lorusso,2 Ana Oaknin,3 Tamar Safra,4 Elizabeth M. Swisher,5 Igor M. Bondarenko,6 Tomasz Huzarski,7 Jaroslav Klat,8 Vladimir Moiseyenko,9 Róbert Póka,10 Luciana S. Viola,11 Chris Tankersley,12 Lara Maloney,12 Sandra Goble,12 Caro Unger,12 Adam Dowson,12 Heidi Giordano,12 Amit M. Oza13 1University College London Cancer Institute, London, UK; 2MITO and Unità di Ginecologia Oncologica, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy; 3Vall d’Hebron University Hospital, Vall d’Hebron Institute of Oncology (VHIO), Barcelona, Spain; 4Sackler School of Medicine, Tel Aviv University and Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; 5University of Washington, Seattle, WA, USA; 6Dnipropetrovsk Medical Academy, City Multiple-Discipline Clinical Hospital, Dnipropetrovsk, Ukraine; 7Private Health Care Innovative Medicine, Grzepnica, Poland; 8University Hospital Ostrava, Ostrava, Czech Republic; 9NN. Petrov Research Institute of Oncology Cancer Center, St. Petersburg, Russian Federation; 10Debrecen University Clinical Center, Debrecen, Hungary; 11Pontifical Catholic University of Rio Grande do Sul, Porto Alegre, Brazil; 12Clovis Oncology, Inc., Boulder, CO, USA; 13Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada INTRODUCTION • In high-grade OC, including fallopian tube and primary peritoneal cancer, ≈18% and ≈7% of -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -

Combining Immune Checkpoint Inhibitors: Established and Emerging Targets and Strategies to Improve Outcomes in Melanoma
King’s Research Portal DOI: 10.3389/fimmu.2019.00453 Document Version Publisher's PDF, also known as Version of record Link to publication record in King's Research Portal Citation for published version (APA): Khair, D. O., Bax, H. J., Mele, S., Crescioli, S., Pellizzari, G., Khiabany, A., Nakamura, M., Harris, R. J., French, E., Hoffmann, R. M., Williams, I. P., Cheung, K. K. A., Thair, B., Beales, C. T., Touizer, E., Signell, A. W., Tasnova, N. L., Spicer, J. F., Josephs, D. H., ... Karagiannis, S. N. (2019). Combining Immune Checkpoint Inhibitors: Established and Emerging Targets and Strategies to Improve Outcomes in Melanoma. Frontiers in Immunology , (MAR), [453]. https://doi.org/10.3389/fimmu.2019.00453 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. •Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. -

Immune Checkpoint Inhibition in DLBCL Immunotherapy: “The Cure Is Inside Us”
Mariano Provencio Servicio de Oncología Médica Hospital Universitario Puerta de Hierro Immune checkpoint inhibition in DLBCL Immunotherapy: “The Cure is Inside Us” § Our immune system prevents or limit infections by foreign antigens expressed in microorganisms (bacteria, viruses, etc.) § Our immune system can also recognize and destroy cancer cells….. • However, cancer cells have developed “escape mechanisms” to avoid their destruction by immune cells…“put the brakes on” • Immuno-Oncology: Find ways of “unleashing” the power of our body’s immune system to treat or prevent cancer…T-lymphocytes (T- cells) Detectives Dendritic cells Killer –T cells Microenvironment antigen (flags) Detectives Dendritic cells Killer –T cells Tumor infiltranting T cell recognizable ags Cor e Algorithms Margin Neo-antigens antigen (flags) 5 6 Scott DW et al. Nature Rev 2014 Strategy approach § Effective immune response: barriers § microenviroment § Activate anti-tumor immune response § inhibitory receptors: blocking antibodies § Nivo, Pembro, Ipi,…(anti PD 1) (CTLA4) § combining 2 checkpoint inhibitors § combining with chemotherapy § activate receptors: agonist § Urelumab § Utumilumab § Varlilumab Strategy approach § Effective immune response: barriers § microenviroment Inactivated effector T cell angiogenesis metabolism Strategy approach PDL-1 Effective immune CTLA-4 response: barriers Lymphoma PDL-1 PD-1 PDL-1 TIM-3 PDL-1 PDL-1 mTOR LAG-3 OXPHOS MHC1 Aerobic glycolysis Interferon gamma EB virus T cell activation antigen presenting cells Strategy approach § -
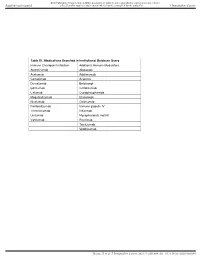
Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezol
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezolizumab Abatacept Avelumab Adalimumab Cemiplimab Anakinra Durvalumab Belatacept Ipilimumab Certolizumab Lirilumab Cyclophosphamide Mogamulizumab Etanercept Nivolumab Golimumab Pembrolizumab Immune globulin IV Tremelimumab Infliximab Urelumab Mycophenolate mofetil Varlilumab Rituximab Tocilizumab Vedolizumab Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S2. Toxicity of additional immune modulators Treatment detail Toxicity Patient 1 day 18,32 Infliximab dosed day 59 P.aeruginosa, S.marcescens pneumonia; died Patient 2 day 9 Infliximab dosed day 44 Febrile neutropenia; P. aeruginosa SBP and day 26 Mycophenolate initiated sepsis; C. albicans fungemia; treated and discharged Patient 8 day 4,11 Infliximab dosed day 14 Disseminated HSV-1; died Patient 26 day 79-128 Infliximab dosed (x7) day 130 E. faecalis, P. aeruginosa bacteremia; died; day 81, 97 Cyclophosphamide dosed Fungal pneumonia on autopsy Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S3. -

On the Horizon: Immuno-Oncology (I-O) Combinations
Immuno-Oncology (I-O) Combinations • Jeffrey A. Sosman, MD • Robert H. Lurie Comprehensive Cancer Center of Northwestern University The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 Stimulatory and Inhibitory Factors in the Cancer-Immunity Cycle Each step of the Cancer-Immunity Cycle requires the coordination of numerous factors, both stimulatory and inhibitory in nature. Stimulatory factors shown in green promote immunity, ... Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Block other co-inhibitory: LAG3, TIM3, KIR, VISTA • Activate co-stimulatory: 4-1BB, OX-40, GITR, CD27, ICOS • Block inhibitory molecules- IDOi, TGFbi, CSF1Ri, anti-IL-6 or anti- IL-10 • Effect trafficking- anti-VEGF, CCL5, CXCR4i • Vaccines- TVEC- oncolytic virus, Neoantigen, other cellular • Adoptive Cellular therapy- TIL, CAR-T cells, TCR T-cells Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Signal Inhibition, BRAF directed (BRAFi+MEKi), MEKi, PI3K inhibition (PTEN effects) • Cytokines- IL-2, IFN a,b,g,, Directed cytokines (FAP-IL-2v or CEA-IL-2v) • Epigenetic modulation- gene expression and EVR expression • Microbiome modification- fecal transplants • Chemotherapy other cytotoxics • Localized Irradiation SBRT, SRS T cells in Tumors Express Multiple Immunoinhibitory Receptors