Standing Committee on Health
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Federal Politics
REPORT FEDERAL POLITICS st DATE FebruaryNUMÉRO1 DE, 2020 PROJET METHODOLOGY METHODOLOGY Web survey using computer-assisted Web interviewing (CAWI) technology. From January 29th to January 30th, 2020 1,501 Canadians, 18 years of age or older, randomly recruited from LEO’s online panel. Using data from the 2016 Census, results were weighted according to age, gender, mother tongue and region in order to ensure a representative sample of the population. No margin of error can be associated with a non-probability sample (Web panel in this case). However for comparative purposes, a probability sample of 1,501 respondents would have a margin of error of ±2.53%, 19 times out of 20. The research results presented here are in full compliance with the CRIC Public Opinion Research Standards and Disclosure Requirements. 2 METHODOLOGY Notes on Reading this Report The numbers presented have been rounded up. However, the numbers before rounding were used to calculate the sums presented and might therefore not correspond to the manual addition of these numbers. In this report, data in bold red characters indicate a significantly lower proportion than that of other respondents. Conversely, data in bold green characters indicate a significantly higher proportion that that of other respondents. A more detailed methodology is presented in the appendix. If you have questions about the data presented in this report, please contact Christian Bourque, Associate and Executive Vice-Present at the following e-mail address: [email protected] 3 FEDERAL VOTING INTENTIONS Q1A/Q1B. If FEDERAL elections were held today, for which political party would you be most likely to vote? Would it be for...? In the event a respondent had no opinion, the following prompting question was asked: Even if you have not yet made up your mind, for which of the following political parties would you be most likely to vote? Would it be for the .. -

Liberal Base 'Less Than Enthusiastic' As PM Trudeau Prepares to Defend
Big Canadian challenge: the world is changing in Health disruptive + powerful + policy transformative briefi ng ways, & we better get HOH pp. 13-31 a grip on it p. 12 p.2 Hill Climbers p.39 THIRTIETH YEAR, NO. 1602 CANADA’S POLITICS AND GOVERNMENT NEWSPAPER MONDAY, FEBRUARY 4, 2019 $5.00 News Liberals News Election 2019 News Foreign policy House sitting last Trudeau opportunity for Liberal base ‘less than ‘masterful’ at Trudeau Liberals soft power, to highlight enthusiastic’ as PM falling short on achievements, hard power, says control the Trudeau prepares to ex-diplomat agenda and the Rowswell message, says a defend four-year record BY PETER MAZEREEUW leading pollster rime Minister Justin Trudeau Phas shown himself to be one to ‘volatile electorate,’ of the best-ever Canadian leaders BY ABBAS RANA at projecting “soft power” on the world stage, but his government’s ith the Liberals and Con- lack of focus on “hard power” servatives running neck W is being called into question as and neck in public opinion polls, say Liberal insiders Canada sits in the crosshairs of the 13-week sitting of the House the world’s two superpowers, says is the last opportunity for the The federal Liberals are heading into the next election with some members of the a former longtime diplomat. Continued on page 35 base feeling upset that the party hasn’t recognized their eff orts, while it has given Continued on page 34 special treatment to a few people with friends in the PMO, say Liberal insiders. Prime News Cybercrime Minister News Canada-China relations Justin Trudeau will RCMP inundated be leading his party into Appointing a the October by cybercrime election to special envoy defend his reports, with government’s a chance for four-year little success in record before ‘moral suasion’ a volatile prosecution, electorate. -

Environmental
Back to normal is still a long way off Gwynne Dyer p. 12 What now of the Michael environmental Harris movement in Canada? p.11 Phil Gurski p. 11 Some MPs donating their salary increases to charities p. 4 THIRTY-FIRST YEAR, NO. 1718 CANADA’S POLITICS AND GOVERNMENT NEWSPAPER MONDAY, APRIL 13, 2020 $5.00 News Remote caucus meetings Analysis Feds’ response Analysis: Did In the time of the pandemic, the feds flip- flop on closing Liberals holding national caucus the border or wearing meetings seven days a week masks amid The Liberals' daily Liberals meetings start with the COVID-19 are using a an update for MPs on new developments outbreak? regular daily and the government's initiatives from BY PETER MAZEREEUW conference Deputy House Leader Kirsty Duncan, he federal government says call for their left, International science and expert advice is Trade Minister Mary T caucus behind its decision to shut the Ng, and Minister border to travellers and its chang- meetings. The of Middle Class ing advice on whether Canadians Prosperity Mona should wear masks amid the CO- Conservatives Fortier. Usually, VID-19 outbreak. While Canada’s a member of the are using top health official pointed to COVID-19 cabinet new science related to using face Zoom and committee, or masks, one expert says there is another cabinet no scientific evidence that could the New minister also joins have informed Canada’s decision them in updating Democrats to close its border on March 16. caucus members. “There is no science about The Hill Times are using whether it works to restrict all photographs by travel into a country,” said Steven GoToMeeting. -

List of Mps on the Hill Names Political Affiliation Constituency
List of MPs on the Hill Names Political Affiliation Constituency Adam Vaughan Liberal Spadina – Fort York, ON Alaina Lockhart Liberal Fundy Royal, NB Ali Ehsassi Liberal Willowdale, ON Alistair MacGregor NDP Cowichan – Malahat – Langford, BC Anthony Housefather Liberal Mount Royal, BC Arnold Viersen Conservative Peace River – Westlock, AB Bill Casey Liberal Cumberland Colchester, NS Bob Benzen Conservative Calgary Heritage, AB Bob Zimmer Conservative Prince George – Peace River – Northern Rockies, BC Carol Hughes NDP Algoma – Manitoulin – Kapuskasing, ON Cathay Wagantall Conservative Yorkton – Melville, SK Cathy McLeod Conservative Kamloops – Thompson – Cariboo, BC Celina Ceasar-Chavannes Liberal Whitby, ON Cheryl Gallant Conservative Renfrew – Nipissing – Pembroke, ON Chris Bittle Liberal St. Catharines, ON Christine Moore NDP Abitibi – Témiscamingue, QC Dan Ruimy Liberal Pitt Meadows – Maple Ridge, BC Dan Van Kesteren Conservative Chatham-Kent – Leamington, ON Dan Vandal Liberal Saint Boniface – Saint Vital, MB Daniel Blaikie NDP Elmwood – Transcona, MB Darrell Samson Liberal Sackville – Preston – Chezzetcook, NS Darren Fisher Liberal Darthmouth – Cole Harbour, NS David Anderson Conservative Cypress Hills – Grasslands, SK David Christopherson NDP Hamilton Centre, ON David Graham Liberal Laurentides – Labelle, QC David Sweet Conservative Flamborough – Glanbrook, ON David Tilson Conservative Dufferin – Caledon, ON David Yurdiga Conservative Fort McMurray – Cold Lake, AB Deborah Schulte Liberal King – Vaughan, ON Earl Dreeshen Conservative -
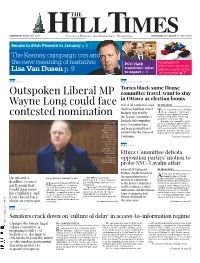
Outspoken Liberal MP Wayne Long Could Face Contested Nomination
THIRTIETH YEAR, NO. 1617 CANADA’S POLITICS AND GOVERNMENT NEWSPAPER WEDNESDAY, MARCH 27, 2019 $5.00 Senate to ditch Phoenix in January p. 5 The Kenney campaign con and the new meaning of narrative: PCO clerk Budget puts off transition: what pharmacare reform, but Lisa Van Dusen feds say it takes the fi rst p. 9 to expect p. 6 ‘concrete steps’ p. 7 News Politics News Parliamentary travel Tories block some House Outspoken Liberal MP committee travel, want to stay in Ottawa as election looms Wayne Long could face Half of all committee trips BY NEIL MOSS that have had their travel he Conservatives are blocking budgets approved by THouse committee travel, say contested nomination some Liberal committee chairs, the Liaison Committee’s but Tory whip Mark Strahl sug- gested it’s only some trips. Budgets Subcommittee A number of committees have Liberal MP had travel budgets approved by Wayne Long since November have the Liaison Committee’s Subcom- has earned a not been granted travel mittee on Committee Budgets, reputation as an but have not made it to the next outspoken MP, authority by the House of step to get travel authority from something two New Brunswick Commons. Continued on page 4 political scientists say should help News him on the Politics campaign trail in a traditionally Conservative Ethics Committee defeats riding. The Hill Times photograph by opposition parties’ motion to Andrew Meade probe SNC-Lavalin aff air Liberal MP Nathaniel BY ABBAS RANA Erskine-Smith described week after the House Justice the opposition parties’ ACommittee shut down its He missed a BY SAMANTHA WRIGHT ALLEN The MP for Saint John- probe of the SNC-Lavalin affair, Rothesay, N.B., is one of about 20 motion as ‘premature,’ the House Ethics Committee also deadline to meet utspoken Liberal MP Wayne Liberal MPs who have yet to be as the Justice Committee defeated an opposition motion Long could face a fi ght for nominated. -

Evidence of the Liaison Committee
43rd PARLIAMENT, 2nd SESSION Liaison Committee EVIDENCE NUMBER 002 PUBLIC PART ONLY - PARTIE PUBLIQUE SEULEMENT Monday, February 22, 2021 Chair: The Honourable Judy A. Sgro 1 Liaison Committee Monday, February 22, 2021 ● (1935) Welcome to all of our new folks who are going to be with us [English] whenever we have our liaison meetings. The Chair (Hon. Judy A. Sgro (Humber River—Black Okay, the committee's in agreement with the agenda. Creek, Lib.)): This is an interesting meeting with all of us here, so we'll all have to have a little bit of patience while we try to move I would invite the clerk to proceed with the election of the vice- along on our agenda tonight. chair. Are there any comments on the proposed agenda? Is the commit‐ David Sweet was the previous vice-chair, but he has stepped tee in favour of the agenda that was sent to you all? down from that role, so we need to elect another vice-chair. I'm not seeing any opposition, so I'll just keep moving forward. The Clerk: Thank you, Madam Chair. We're joined, of course, by Robert Benoit, the principal clerk, Pursuant to Standing Order 106(2), the vice-chair must be a who is going to guide us through our committee work tonight. member of the official opposition. Mr. Benoit, do you want to say a few words before we start the I'm now ready to receive motions for the vice-chair. meeting? Mrs. Kelly Block (Carlton Trail—Eagle Creek, CPC): I nomi‐ The Clerk of the Committee (Mr. -

Angry Birds: Twitter Harassment of Canadian Female Politicians By
Angry Birds: Twitter Harassment of Canadian Female Politicians By Jess Ann Gordon Submitted to the Faculty of Extension University of Alberta In partial fulfillment of the requirements for the degree of Master of Arts in Communications and Technology August 5, 2019 2 Acknowledgments Written with gratitude on the unceded traditional territories of the Skwxw�7mesh (Squamish), Səl̓ �lwətaʔ/Selilwitulh (Tsleil-Waututh), and xʷməθkʷəy̓əm (Musqueam) Nations, and on Treaty 6 territory, the traditional lands of diverse Indigenous peoples including the Cree, Blackfoot, Métis, Nakota Sioux, Iroquois, Dene, Ojibway, Saulteaux, Anishinaabe, Inuit, and many others. I would like to take this opportunity to thank my friends, family, cohort colleagues, and professors who contributed to this project. Thank you to my project supervisor, Dr. Gordon Gow, for his steadying support throughout the project and the many valuable suggestions. Thank you as well to Dr. Stanley Varnhagen, who provided invaluable advice on the design and content of the survey. I am grateful to both Dr. Gow and Dr. Varnhagen for sharing their expertise and guidance to help bring this project to life. Thank you to my guinea pigs, who helped me to identify opportunities and errors in the draft version of the survey: Natalie Crawford Cox, Lana Cuthbertson, Kenzie Gordon, Ross Gordon, Amanda Henry, Lucie Martineau, Kory Mathewson, and Ian Moore. Thank you to my MACT 2017 cohort colleagues and professors their support and encouragement. Particularly, I’d like to thank Ryan O’Byrne for helping me to clarify the project concept in its infant stages, and for being a steadfast cheerleader and friend throughout this project and the entire MACT program. -

Office of the County Warden April 26, 2021 the Right Honourable Justin
Office of the County Warden Telephone: 519-845-0801 789 Broadway Street, Box 3000 Toll-free: 1-866-324-6912 Wyoming, ON N0N 1T0 Fax: 519-845-3160 April 26, 2021 The Right Honourable Justin Trudeau, P.C., MP Prime Minister of Canada Office of the Prime Minister 80 Wellington Street Ottawa, ON K1A 0A2 Sent via email: [email protected] The Honourable Chrystia Freeland, P.C., M.P. Deputy Prime Minister and Minister of Finance Privy Council Office, Room 1000 80 Sparks Street Ottawa, ON K1A 0A3 Sent via email: [email protected] Dear Prime Minister Trudeau and Deputy Prime Minister Freeland: Re: Basic Income for Income Security during COVID-19 Pandemic and Beyond At its meeting held on February 3rd, 2021, Lambton County Council received correspondence to the federal government from the Thunder Bay District Health Unit dated November 20, 2020 with respect to using a basic income to address food security. This letter is intended to express our support for these efforts to provide income solutions to reduce food insecurity. Income is one of the strongest predictors of health, and it makes sense that focusing on population health interventions to address socioeconomic factors will impact health outcomes far greater than individual focused interventions. Prior to COVID-19, 8% of Lambton County residents reported moderate or severe food insecurity: experiencing actual issues with procuring an adequate quality or quantity of food, or worrying about the source of their food. Since COVID-19, this pre-existing issue has become more apparent and worrisome with Statistics Canada reporting an increase to 14.6% or 1 in 5 households. -

LOBBY MONIT R the 43Rd Parliament: a Guide to Mps’ Personal and Professional Interests Divided by Portfolios
THE LOBBY MONIT R The 43rd Parliament: a guide to MPs’ personal and professional interests divided by portfolios Canada currently has a minority Liberal government, which is composed of 157 Liberal MPs, 121 Conservative MPs, 32 Bloc Québécois MPs, 24 NDP MPs, as well as three Green MPs and one Independent MP. The following lists offer a breakdown of which MPs have backgrounds in the various portfolios on Parliament Hill. This information is based on MPs’ official party biographies and parliamentary committee experience. Compiled by Jesse Cnockaert THE LOBBY The 43rd Parliament: a guide to MPs’ personal and professional interests divided by portfolios MONIT R Agriculture Canadian Heritage Children and Youth Education Sébastien Lemire Caroline Desbiens Kristina Michaud Lenore Zann Louis Plamondon Martin Champoux Yves-François Blanchet Geoff Regan Yves Perron Marilène Gill Gary Anandasangaree Simon Marcil Justin Trudeau Claude DeBellefeuille Julie Dzerowicz Scott Simms Filomena Tassi Sean Casey Lyne Bessette Helena Jaczek Andy Fillmore Gary Anandasangaree Mona Fortier Lawrence MacAulay Darrell Samson Justin Trudeau Harjit Sajjan Wayne Easter Wayne Long Jean-Yves Duclos Mary Ng Pat Finnigan Mélanie Joly Patricia Lattanzio Shaun Chen Marie-Claude Bibeau Yasmin Ratansi Peter Schiefke Kevin Lamoureux Francis Drouin Gary Anandasangaree Mark Holland Lloyd Longfield Soraya Martinez Bardish Chagger Pablo Rodriguez Ahmed Hussen Francis Scarpaleggia Karina Gould Jagdeep Sahota Steven Guilbeault Filomena Tassi Kevin Waugh Richard Lehoux Justin Trudeau -

Members of Parliament with Anti-Choice Stance, and Unknown
Members of Parliament with an Anti-choice Stance October 16, 2019 By Abortion Rights Coalition of Canada History: After 2015 election (last updated May 2016) Prior to 2015 election (last updated Feb 2015) After May 2011 election (last updated Sept 2012) After 2008 election (last updated April 2011) Past sources are listed at History links. Unknown or Party Total MPs Anti-choice MPs** Pro-choice MPs*** Indeterminate Stance Liberal 177 6* (3.5%) 170* (96%) 1 Conservative 95 76 (80%) 8 (8%) 11 (12%) NDP 39 0 39 0 Bloc Quebecois 10 0 10 0 Independent 8 1 6 1 Green 2 0 2 0 Co-operative 1 0 1 0 Commonwealth Federation People’s Party 1 1 0 Vacant (5) Total 333 84 (25%) 236 (71%) 13 (4%) (not incl. vacant) (Excluding Libs/PPC: 23%) *All Liberal MPs have agreed and will be required to vote pro-choice on any abortion-related bills/motions. Also, Trudeau will not likely allow anti-choice MPs to introduce their own bills/motions, or publicly advocate against abortion rights. Therefore, these MPs should not pose any threat, although they should be monitored. Likewise, Liberal MPs not on this 2014 pro-choice list may warrant monitoring to ensure they adhere to the party’s pro-choice policy. Oct 2016, Bill C-225 update: All 7 Liberals with previous anti-choice records voted No, except for John McKay who did not vote. **Anti-choice MPs are designated as anti-choice based on at least one of these reasons: • Voted in favour of Bill C-225, and/or Bill C-484, and/or Bill C-510, and/or Motion 312 • Opposed the Order of Canada for Dr. -

Health on the Hill: Parliament Mulls Vaping Regulations
NEWS Health on the Hill: Parliament mulls vaping regulations n Cite as: CMAJ 2018 February 26;190:E232-3. doi: 10.1503/cmaj.109-5568 Posted on cmajnews.com on Feb.8, 2018. arliament debated new vaping rules and other public health bills in its first week back after Pwinter break. Vaping and plain packaging Bill S-5 would update existing tobacco laws to regulate vaping products in much the same way as other tobacco products, including provisions to restrict sales to youth and ban flavoured products. The bill proposes to allow limited promotion of vaping products as a tool to help people quit smoking; however, it will pro- hibit claims of health benefits. “The key message that needs to be emphasized is that while scientific knowledge is still evolving on the issue, there is much more work to be done,” said Kevin Lamoureux, Parliamentary Secretary to the Leader of the Government. “It is clear that vaping products may bring public health bene- fits, if they reduce tobacco-related death NicolasMcComber/iStock and disease by helping smokers quit or A Senate bill to restrict youth access to vaping products is before the Standing Committee on Health. switch completely to a less harmful source of nicotine, but it may also harm Taxation Joël Lightbound, Parliamentary Secretary young people, in particular.” Conservative MP Marilyn Gladu high- to the Minister of Finance, said the reforms The bill also would introduce plain lighted the “unintended consequences” are necessary to “ensure a level playing packaging for tobacco and vaping prod- of upcoming small-business tax reforms, field,” and will affect only the richest 3% of ucts, replacing branding with health dis- arguing that people who own corpora- private corporations. -

FEDERAL GOVERNMENT Quick Reference Guide
FEDERAL GOVERNMENT Quick Reference Guide: Key Officials and Opposition Critics PARLIMENTARY DEPUTY MINISTRY MINISTER CHIEF OF STAFF CONSERVATIVE CRITIC NDP CRITIC ASSISTANT MINISTER Celina Caesar- PRIME MINISTER OF CANADA Rt. Hon Justin Trudeau Katie Telford Chavannes Agriculture and Agri-Food Hon. Lawrence MacAulay Jean-Claude Poissant Andrea Lyon Chris Warkentin Ruth Ellen Brosseau Mary Jean McFall Hon. Harjit Sajjan John Forster James Bezan Randall Garrison National Defence Brian Bohunicky John McKay Democratic Institutions Hon. Maryam Monsef Mark Holland Scott Reid Sheri Benson Maxime Dea Employment Workforce Development and Hon. MaryAnn Mihychuk Rodger Cuzner Lori Sterling Gérard Deltell Niki Ashton Labour – Gov’t Canada Labour Program Matthew Mitschke Environment and Climate Change Hon. Catherine McKenna Jonathan Wilkinson Michael Martin Hon. Ed Fast Nathan Cullen Marlo Raynolds Families, Children and Social Development Hon. Jean-Yves Duclos Terry Duguid Karen Vecchio Brigitte Sansoucy Josee Duplessis Ian Shugart Francois-Philippe Finance Hon. Bill Morneau Richard Paul Rochon Hon. Lisa Raitt Guy Caron Champagne Maksymetz Fisheries and Oceans, and Canadian Hon. Hunter Tootoo Serge Cormier Matthew King Mark Strahl Fin Donnelly Coastguard Hon. Tony Clement Hélène Laverdière Pamela Goldsmith- Foreign Affairs Hon. Stéphane Dion Daniel Jean Julian Ovens Jones Omar Alghabra (Consular Affairs) Simon Health Hon. Jane Philpott Kamal Khera Hon. K. Kellie Leitch Don Davies Genevieve Hinse Kennedy Hon. Peter Van Canadian Heritage Hon. Mélanie