Deposit Classification Scheme for the Critical Minerals Mapping Initiative Global Geochemical Database
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineralogy and Geochemistry Study of Ree Minerals in Host Rocks in Iic Iron Deposit, Bafgh Mineral Area, Central Iran
GEOSABERES: Revista de Estudos Geoeducacionais ISSN: 2178-0463 [email protected] Universidade Federal do Ceará Brasil MINERALOGY AND GEOCHEMISTRY STUDY OF REE MINERALS IN HOST ROCKS IN IIC IRON DEPOSIT, BAFGH MINERAL AREA, CENTRAL IRAN SHIRNAVARD SHIRAZI, MANSOUREH; LOTFI, MOHAMMAD; NEZAFATI, NIMA; GOURABJERIPOUR, ARASH MINERALOGY AND GEOCHEMISTRY STUDY OF REE MINERALS IN HOST ROCKS IN IIC IRON DEPOSIT, BAFGH MINERAL AREA, CENTRAL IRAN GEOSABERES: Revista de Estudos Geoeducacionais, vol. 11, 2020 Universidade Federal do Ceará, Brasil Available in: https://www.redalyc.org/articulo.oa?id=552861694014 DOI: https://doi.org/10.26895/geosaberes.v11i0.909 This work is licensed under Creative Commons Attribution-NonCommercial 4.0 International. PDF generated from XML JATS4R by Redalyc Project academic non-profit, developed under the open access initiative MANSOUREH SHIRNAVARD SHIRAZI, et al. MINERALOGY AND GEOCHEMISTRY STUDY OF REE MINERALS IN HOST ROC... MINERALOGY AND GEOCHEMISTRY STUDY OF REE MINERALS IN HOST ROCKS IN IIC IRON DEPOSIT, BAFGH MINERAL AREA, CENTRAL IRAN ESTUDO DE MINERALOGIA E GEOQUÍMICA DE MINERAIS REE EM ROCHAS HOSPEDEIRAS NO DEPÓSITO DE FERRO DA IIC, ÁREA MINERAL DE BAFGH, IRÃ CENTRAL ESTUDIO DE MINERALOGÍA Y GEOQUÍMICA DE MINERALES REE EN ROCAS HOSPEDANTES DE DEPÓSITOS DE HIERRO DE LA CII, ÁREA MINERAL DE BAFGH, IRÁN CENTRAL MANSOUREH SHIRNAVARD SHIRAZI DOI: https://doi.org/10.26895/geosaberes.v11i0.909 Islamic Azad University, Irán Redalyc: https://www.redalyc.org/articulo.oa? [email protected] id=552861694014 http://orcid.org/0000-0001-9242-0341 -

Attributes of Skaergaard-Type PGE Reefs
Attributes of Skaergaard-Type PGE Reefs James D. Miller, Jr.1 and Jens C. Ø. Andersen2 1Minnesota Geological Survey, c/o NRRI, University of Minnesota-Duluth, 5013 Miller Trunk Hwy., Duluth, MN, 55811 , USA 2Camborne School of Mines, University of Exeter, Redruth, Cornwall, TR15 3SE UK e-mail: [email protected], [email protected] Stratiform, platinum group element (PGE) peridotite, pyroxenite, and dunite are usually deposits have long been known to occur in quantitatively minor. When well-differentiated, ultramafic-mafic intrusive complexes such as such intrusions display a simplified cumulus Bushveld and Stillwater (Naldrett, 1989b). stratigraphy following the scheme: Ol or Pl only-> Commonly known as PGE reefs, such deposits are Ol + Pl -> Pl + Cpx + FeOx ± Ol -> Pl + Cpx + typically found near the transition from ultramafic FeOx + Ol + Ap. They have a strong cryptic to mafic cumulates where they occur as 1-3 meter layering towards iron-enrichment indicative of thick intervals enriched in PGEs (1-20 ppm) and Fenner-type differentiation. They differ from trace to moderate amounts of sulfide (0.5-5 wt %). classic PGE reef-bearing intrusions by lacking a However, relatively recent discoveries have significant ultramafic component (early olivine- demonstrated that potentially economic stratiform only crystallization is minor to absent in most PGE mineralization may also occur in tholeiitic tholeiitic intrusions) and by being poor in mafic layered intrusions. Au- and PGE-bearing orthopyroxene (inverted pigeonite is rarely a layers -

Monchegorsk Layered Intrusion, Fennoscandian Shield)
minerals Article Chromite Mineralization in the Sopcheozero Deposit (Monchegorsk Layered Intrusion, Fennoscandian Shield) Artem V. Mokrushin 1,* and Valery F. Smol’kin 2 1 Geological Institute—Subdivision of the Federal Research Centre “Kola Science Centre of the Russian Academy of Sciences”, 14 Fersman Street, 184209 Apatity, Russia 2 Vernadsky State Geological Museum of the Russian Academy of Sciences, 11/11 Mokhovaya Street, 125009 Moscow, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-(902)133-39-95 Abstract: In 1990, the Sopcheozero Cr deposit was discovered in the Monchegorsk Paleoprotero- zoic layered mafic-ultramafic layered intrusion (Monchepluton). This stratiform early-magmatic deposit occurs in the middle part of the Dunite Block, which is a member of the Monchepluton layered series. The Cr2O3 average-weighted content in ordinary and rich ores of the deposit is 16.65 and 38.76 wt.%, respectively, at gradually changing concentrations within the rich, ordinary and poor ore types and ore body in general. The ores of the Sopcheozero deposit, having a ratio of Cr2O3/FeOtotal = 0.9–1.7, can serve as raw materials for the refractory and chemical industries. The ore Cr-spinel (magnochromite and magnoalumochromite) is associated with highly magnesian olivine (96–98 Fo) rich in Ni (0.4–1.1 wt.%). It confirms a low S content in the melt and complies with the low oxygen fugacity. The coexisting Cr-spinel-olivine pairs crystallized at temperatures ◦ from 1258 to 1163 C, with accessory Cr-spinel crystallizing at relatively low, while ore Cr-spinel at higher temperatures. The host rock and ore distinguish with widespread plastic deformations of ◦ Citation: Mokrushin, A.V.; Smol’kin, olivine at the postcrystallization phase under conditions of high temperature (above 400 C) and V.F. -

Oxygen and Iron Isotope Systematics of the Grängesberg Mining District (GMD), Central Sweden
Oxygen and Iron Isotope Systematics Examensarbete vid Institutionen för geovetenskaper of the Grängesberg Mining District ISSN 1650-6553 Nr 251 (GMD), Central Sweden Franz Weis Oxygen and Iron Isotope Systematics of the Grängesberg Mining District Iron is the most important metal for modern industry and Sweden is (GMD), Central Sweden the number one iron producer in Europe. The main sources for iron ore in Sweden are the apatite-iron oxide deposits of the “Kiruna-type”, named after the iconic Kiruna ore deposit in Northern Sweden. The genesis of this ore type is, however, not fully understood and various schools of thought exist, being broadly divided into “ortho-magmatic” versus the “hydrothermal replacement” approaches. This study focuses on the origin of apatite-iron oxide ore of the Grängesberg Mining District (GMD) in Central Sweden, one of the largest iron reserves in Sweden, employing oxygen and iron isotope analyses on Franz Weis massive, vein and disseminated GMD magnetite, quartz and meta- volcanic host rocks. As a reference, oxygen and iron isotopes of magnetites from other Swedish and international iron ores as well as from various international volcanic materials were also analysed. These additional samples included both “ortho-magmatic” and “hydrothermal” magnetites and thus represent a basis for a comparative analysis with the GMD ore. The combined data and the derived temperatures support a scenario that is consistent with the GMD apatite-iron oxides having originated dominantly (ca. 87 %) through ortho-magmatic processes with magnetite crystallisation from oxide-rich intermediate magmas and magmatic fluids at temperatures between of 600 °C to 900 °C. -

Insights on the Effects of the Hydrothermal Alteration in the El Laco Magnetite Deposit (Chile) / FRANCISCO VELASCO (1.�), FERNANDO TORNOS (2)
macla nº 16. junio ‘12 210 revista de la sociedad española de mineralogía Insights on the Effects of the Hydrothermal Alteration in the El Laco Magnetite Deposit (Chile) / FRANCISCO VELASCO (1.1), FERNANDO TORNOS (2) (1) Dpto. de Mineralogía y Petrología.Universidad del País Vasco UPV/EHU, Sarriena s/n, 0 Leioa, Spain. (2) Instituto Geológico y Minero de España, Madrid, Spain. INTRODUCCIÓN The understanding of the origin of the recent (ca. 2 Ma) El Laco deposit (Fig. 1), with near 1 Gt of almost pure magnetite/hematite, is considered critical for the interpretation of the Kiruna type magnetite-apatite style of mineralization, an end-member of the IOCG group of deposits. Despite the abundant studies conducted in the last decades on El Laco, with little erosion, well preserved volcanic features and excellent conditions of exposure, there is no agreement between models that support a genesis related to the hydrothermal replacement of preexisting andesitic rocks (Rhodes & Oreskes, 1999; Rhodes et al., 1999) and those which interpret the deposit as magmatic flows and dikes product of the crystallization of an iron oxide melt (Frutos & Oyarzun, 1975; Nyström & Henríquez, 1994; Naslund et al., 2002; Henríquez et al., 2003; Tornos et al., fig. 1 Schematic geological map of the magnetite orebodies (black) at the El Laco district hosted in the Plio- 2011). To solve this fascinating Pleistocene andesitic volcanic arc, northern Chile (modified from Frutos M Oyarzun, 1PQR).! controversy is crucial to understand the problem from a global point of view, host rocks. Except for some dikes, most crystals (size mm to several cm) integrating geological and geochemical of the magnetite orebodies (Laco Sur, intergrown with prismatic-acicular data of the magmatic and hydrothermal Laco Norte, S. -

Geology 222 Laboratory Project Layered Mafic Intrusions
Geology 222 Laboratory Project Layered Mafic Intrusions Layered mafic intrusions present an unusual record of a natural crystallization experiment. In an ideal case, a magma chamber is filled with a mafic liquid of relatively low viscosity. Crystals of sufficient density that grow from this liquid settle to the bottom of the magma chamber as they grow. Layers of crystals are formed yielding a “magmatic sedimentary rock”. The first-formed crystals are on the bottom of the magma chamber and later-formed crystals are stratigraphically above earlier-formed crystals. If one looks at the compositions of the crystals in the layers, changes that occur during the solidification of the magma can be discovered. Actual layered mafic intrusions are rarely as simple as the idealized model. Plagioclase commonly sinks, rather than floats as its density suggests it would do. Crystal settling can be rhythmic rather than continuous. Density currents can develop and lead to cross-bedding. Some (intercumulus) liquid can be trapped as a pore fluid among the settled crystals and may possibly be squeezed out as the crystal layers are compressed. Additional batches of magma can be added to the magma chamber over time, changing the composition of the evolving liquid. The lab assignment is to look carefully at rocks from one layered mafic intrusions for which we have thin sections (Bushveld, Kiglapait, and Stillwater). You should describe fully three thin sections from the intrusion of your choice. Chose thin sections that are not similar to one another. Identify the minerals. Give a mode. Include in your report photomicrographs or sketches of textures that you see. -

Resources in the Upper Zone of the Bushveld Complex, South Africa
Papers and Proceedings of the Royal Society of Tasmania, Volume 150(1), 2016 15 Fe-Ti-V-(P) RESOURCES IN THE UPPER ZONE OF THE BUSHVELD COMPLEX, SOUTH AFRICA by L.A. Fischer and Q. Yuan (with four text-figures and two plates) Fischer, L.A. & Yuan, Q. 2016 (31:viii): Fe-Ti-V-(P) resources in the Upper Zone of the Bushveld Complex, South Africa. Papers and Proceedings of the Royal Society of Tasmania 150(1): 15–22. https://doi.org/10.26749/rstpp.150.1.15 ISSN 0080-4703. Institut für Mineralogie, Leibniz Universität Hannover, 30167 Hannover, Germany, and School of Physical Sciences, University of Tasmania, Hobart, Tasmania 7001, Australia (LAF*); Faculty of Earth Resources, China University of Geosciences, Wuhan 430074, China, and Department of Geology, University of Liege, 4000 Sart Tilman, Belgium (QY). *Author for correspondence. Email: [email protected] The Bushveld Complex in South Africa is the largest layered intrusion on Earth. Its upper part is known for huge resources of iron, tita- nium, vanadium and phosphorus. Associated with the layered character of the rocks, these resources are enriched at certain levels of the intrusion, which makes it important to understand the formation processes of those layers. In this paper we give an introduction and overview of recent debates and challenges. Key Words: layered intrusion, Bushveld, resources. INTRODUCTION Fe, Ti, V and P never or only rarely occur as native elements in nature. Minerals, rich in one or more of these Iron (Fe), titanium (Ti) and vanadium (V) are important elements are mined and their components are extracted. -
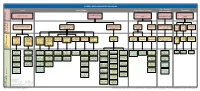
Komatiite Hosted Tree
LAYERED INTRUSION-HOSTED VANADIUM Trap - Chemical/ Source Active Pathway Trap - Chemical Scrubber Preservation physical scrubber Transporting large volumes of Long-lived fractionation to produce Fe-oxide Formation of oxidised seams within Exhumation and preservation of vanadium Voluminous mantle melting mantle-derived magma through the saturation a layered mafic-ultramafic intrusion orebodies crust Critical Critical Process : ? Processes within the magma Mantle plumes to transport large Intracontinental rift zones where Pooling of mantle Stable Preservation High-degree mantle melting to produce Delayed onset of Fe- chamber? Density settling, magma Weathering of volumes of mantle-derived magma extensional forces produced passive derived magma in plateaus of the upper voluminous mafic-ultramafic magmas oxide saturation mixing, pressure changes, liquid magnetite layers through the crust melting of the mantle the crust with slow parts of immiscibility rates of layered erosion intrusions Constituent ProcessConstituent What are the processes that that processes are the What critical process the control Archean granite Archean granite Domal uplift Sedimentary basins Volcanic rifted Plateaus Large Igneous Large Igneous Triple junctions Kimberlites and Rocks coeval with Layered mafic- Magnetitite-ilmenite chromatite greenstone greenstone formed by caused by margins that may and areas Tilted Vanadomaghemite Provinces (LIPs) - Provinces (LIPs) - where mantle carbonatites that or indicators of ultramafic Dykes and sills Tholeiitic magmas layers within -

Platinum-Group Minerals in Chromitites of the Niquelândia
Minerals 2012, 2, 365-384; doi:10.3390/min2040365 OPEN ACCESS minerals ISSN 2075-163X www.mdpi.com/journal/minerals Article Platinum-Group Minerals in Chromitites of the Niquelândia Layered Intrusion (Central Goias, Brazil): Their Magmatic Origin and Low-Temperature Reworking during Serpentinization and Lateritic Weathering Giorgio Garuti 1,*, Federica Zaccarini 1, Joaquin A. Proenza 2, Oskar A. R. Thalhammer 1 and Nelson Angeli 3 1 Department of Applied Geosciences and Geophysics, Montan Universität Leoben, Leoben 8700, Austria; E-Mails: [email protected] (F.Z.); [email protected] (O.A.R.T.) 2 Departament of Crystallography, Mineralogy and Ore Deposits, Faculty of Geology, Universitat de Barcelona, Barcelona 08028, Spain; E-Mail: [email protected] 3 Departament of Petrology and Metallogeny, Universidade Estadual Paulista, Rio Claro 13500, SP, Brazil; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +43-3842-402-6218; Fax: +43-3842-402-6202. Received: 3 September 2012; in revised form: 19 October 2012 / Accepted: 19 October 2012 / Published: 30 October 2012 Abstract: A variety of platinum-group-minerals (PGM) have been found to occur associated with the chromitite and dunite layers in the Niquelândia igneous complex. Two genetically distinct populations of PGM have been identified corresponding to phases crystallized at high temperatures (primary), and others formed or modified during post-magmatic serpentinization and lateritic -

Mineral Chemistry of Magnetite from Magnetite- Apatite Mineralization and Their Host Rocks: Examples from Kiruna, Sweden and El Laco, Chile
Mineral chemistry of magnetite from magnetite- apatite mineralization and their host rocks: Examples from Kiruna, Sweden and El Laco, Chile Shannon G. Broughm A thesis submitted to the Department of Earth Sciences in partial fulfillment of the requirements for the degree of Master of Science. Memorial University of Newfoundland Abstract Magnetite-apatite deposits, sometimes referred to as Kiruna-type deposits, are major producers of iron ore that dominantly consist of the mineral magnetite (nominally 2+ 3+ [Fe Fe 2]O4). It remains unclear whether magnetite-apatite deposits are of hydrothermal or magmatic origin, or a combination of those two processes, and this has been a subject of debate for over a century. Magnetite is sensitive to the physicochemical conditions in which it crystallizes (such as element availability, temperature, pH, fO2, and fS2) and may contain distinct trace element concentrations depending on the growing environment. These properties make magnetite potentially a useful geochemical indicator for understanding the genesis of magnetite-apatite mineralization. The samples used in this study are from precisely known geographic locations and geologic environments in the world class districts of Kiruna and the Atacama Desert and their associated, sometimes hydrothermally altered, host rocks. Trace element analyses results of magnetite from the Kiruna area in the Norrbotten region of northern Sweden, and the El Laco and Láscar volcanoes in the Atacama Desert of northeastern Chile, were evaluated using mineral deposit-type and magmatic vs. hydrothermally derived magnetite discrimination diagrams. The objectives of this study are to critically evaluate the practical use and limitations of these discrimination diagrams with the goal of determining if the trace element chemistry of magnetite can be used to resolve if magnetite-apatite deposits form in a hydrothermal or magmatic environment, or a combination of those two processes. -

Global Correlation of Oxygen and Iron Isotope on Kiruna-Type Ap-Fe-Ox Ores
Global correlation of oxygen and iron isotope on Kiruna-type Ap-Fe-Ox ores Valentin R. Troll, Franz Weis, Erik Jonsson, Ulf B. Andersson , Chris Harris, Afshin Majidi, Karin Högdahl, Marc-Alban Millet, Sakthi Saravanan, Ellen Koijman, Katarina P. Nilsson Iron is master of them all • Despite the need for REE, iron is still the number 1 metal for modern industry…and will remain so for some time (e.g. USGS) • Kiruna-type Ap-Fe-oxide ores are the dominant source of industrially used iron in Europe • ….and Sweden is the country with the dominant concentration of Kiruna – type ore deposits in Europe What are apatite-iron- oxide ores? • Also referred to as the ”Kiruna-type”. Often massive magnetite associated with apatite • Grouped together with IOCG-deposits • Usually associated with subduction zones and extensional settings • Form lense-shaped or disc-like ore bodies • Occur from Paleoproterozoic (e.g. Kiruna), through Proterozoic (Bafq) to Quaternary (e.g. El Laco) What are apatite-iron- oxide ores? • About 355 deposits and prospects worldwide • Contain low-Ti magnetite as main ore mineral and F-rich apatite. Hematite may be present • Known for large sizes and high grades (e.g. Kiruna, pre- mining reserve 2 billion tons, grade > 60%) How do apatite-iron-oxide ores form? • Their origin is not yet fully understood and a debate has been going on for over 100 years. Two broad schools of thought exist: Orthomagmatic ore formation (high-T magmatic) Hydrothermal ore formation (low-T fluids and associated replacement) Aim: Investigate the origin of the massive apatite- iron-oxide ores from Sweden and elsewhere, using stable isotopes of iron and oxygen – the main elements in magnetite Hypothesis Magnetite that formed from magma should be in equilibrium with a magmatic source δ-value (magma or magmatic fluid) as fractionation temperatures should lie in the magmatic range. -

Mineralisation in Layered Mafic-Ultramafic Intrusions
1 Mineralisation in Layered Mafic-Ultramafic Intrusions 2 3 Hannah S. R. Hughes1, Jens C. Ø. Andersen1, Brian O’Driscoll2 4 1Camborne School of Mines, College of Engineering, Mathematics and Physical Sciences, University 5 of Exeter, Tremough Campus, Penryn, Cornwall, TR10 9FE, UK 6 2Department of Earth and Environmental Sciences, University of Manchester, Oxford Road, 7 Manchester, M13 9PL, UK 8 9 10 See Also: Economic Geology, Layered Intrusions, Igneous Rocks, Igneous Processes, Mining Geology, 11 Mantle, Mantle Plumes, Minerals: Sulphides. 12 13 1 14 1. Introduction 15 Mineral deposits form in mafic-ultramafic layered intrusions (LMI) through a variety of magmatic 16 processes. Chromium, platinum-group elements (PGE) and nickel (Ni) are almost exclusively extracted 17 from mafic-ultramafic rocks (Table 1). LMI also host metals such as gold (Au), copper (Cu), cobalt (Co), 18 vanadium (V), titanium (Ti) and scandium (Sc). Mafic-ultramafic rocks are major sources of dimension 19 stone and aggregate, and increasingly, carbon capture and storage may generate significant demands 20 for Mg-silicate (olivine and associated minerals). 21 Chromium, Ti and V are extracted from the oxide minerals chromite, ilmenite and titanomagnetite. 22 Economic concentrations require several tens of percent of the host minerals, and exploration can 23 therefore utilize remote sensing. Chromite is the only naturally-occurring source of Cr that can be 24 economically extracted (Table 1). Vanadium is extracted from titanomagnetite and titanium from 25 ilmenite (Table 1). In some mines, Fe is recovered as a by-product. Chromite forms exclusively in 26 primitive mafic-ultramafic rocks, while titanomagnetite and ilmenite are more common in the evolved 27 parts of LMI.