Chapter 3 Cytoskeleton
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Large-Scale Opening of Utrophints Tandem Calponin Homology (CH
Large-scale opening of utrophin’s tandem calponin homology (CH) domains upon actin binding by an induced-fit mechanism Ava Y. Lin, Ewa Prochniewicz, Zachary M. James, Bengt Svensson, and David D. Thomas1 Department of Biochemistry, Molecular Biology and Biophysics, University of Minnesota, Minneapolis, MN 55455 Edited by James A. Spudich, Stanford University School of Medicine, Stanford, CA, and approved June 20, 2011 (received for review April 21, 2011) We have used site-directed spin labeling and pulsed electron has prevented the development of a reliable structural model for paramagnetic resonance to resolve a controversy concerning the any of these complexes. A major unresolved question concerns structure of the utrophin–actin complex, with implications for the the relative disposition of the tandem CH domains (CH1 and pathophysiology of muscular dystrophy. Utrophin is a homolog of CH2) (9, 10). Crystal structures of the tandem CH domains dystrophin, the defective protein in Duchenne and Becker muscular showed a closed conformation for fimbrin (11) and α-actinin (12), dystrophies, and therapeutic utrophin derivatives are currently but an open conformation for both utrophin (Utr261) (Fig. 1A) being developed. Both proteins have a pair of N-terminal calponin and dystrophin (Dys246) (16). The crystal structure of Utr261 homology (CH) domains that are important for actin binding. suggests that the central helical region connecting CH1 and CH2 Although there is a crystal structure of the utrophin actin-binding is highly flexible. Even for α-actinin, which has a closed crystal domain, electron microscopy of the actin-bound complexes has structure, computational analysis suggests the potential for a high produced two very different structural models, in which the CH do- degree of dynamic flexibility that facilitates actin binding (17). -

Biomarkers in Tbi and Abi: Present, Future, and Going Nowhere
BIOMARKERS IN TBI AND ABI: PRESENT, FUTURE, AND GOING NOWHERE GABRIEL NEWMAN, PH.D., DIRECTOR: THE NEUROSCIENCE TEAM, TOWSON Disclosures: • No ties to drug companies, labs, or medical equipment companies • Studies being conducted by author at present are in area of Neuromoduation as an intervention in Autism, and Photobiomodulation as an intervention in TBI and ABI, not specifically in reliability of biomarkers for these conditions • Author of presentation is primarily a clinician in practice (70%), not researcher (30%) Lab assays: 1. MEG3 and Interluekin: Drops in MEG3 and rise in interleukin-1β (IL-1β), IL-6, and IL-8 correlate with poor prognosis in TBI. Thought to be specific to TBI, but research will tell… (Shao et al, May 2019, Eur Rev Med Pharmacol Sci). High likelihood for good use in future. Relatively easy to order. 2. S100β: calcium binding protein found in astrocytes, responsible for regulating intracellular levels of calcium; not brain specific, thus it shows up in injury not involving TBI. Must be considered along with trauma history. Also, must be taken an hour after concussion. Problem: Hard to obtain because of time limit. – probably going nowhere (Linda Papa, MD, MSc., Sports Med Arthrosc. 2016 Sep; 24(3): 108–115) 3. Glial Fibrillary Acid Protein (GFAP): Glial Fibrillary Acidic Protein (GFAP) is a promising, brain-specific glial-derived biomarker for MTBI in adults and children. GFAP is released in highly increased amounts into blood serum within an hour of an mTBI injury, and can remain elevated for several days after injury. More feasible, given extended detectability. Papa L, Mittal MK, Ramirez J, et al.; . -

Defining Functional Interactions During Biogenesis of Epithelial Junctions
ARTICLE Received 11 Dec 2015 | Accepted 13 Oct 2016 | Published 6 Dec 2016 | Updated 5 Jan 2017 DOI: 10.1038/ncomms13542 OPEN Defining functional interactions during biogenesis of epithelial junctions J.C. Erasmus1,*, S. Bruche1,*,w, L. Pizarro1,2,*, N. Maimari1,3,*, T. Poggioli1,w, C. Tomlinson4,J.Lees5, I. Zalivina1,w, A. Wheeler1,w, A. Alberts6, A. Russo2 & V.M.M. Braga1 In spite of extensive recent progress, a comprehensive understanding of how actin cytoskeleton remodelling supports stable junctions remains to be established. Here we design a platform that integrates actin functions with optimized phenotypic clustering and identify new cytoskeletal proteins, their functional hierarchy and pathways that modulate E-cadherin adhesion. Depletion of EEF1A, an actin bundling protein, increases E-cadherin levels at junctions without a corresponding reinforcement of cell–cell contacts. This unexpected result reflects a more dynamic and mobile junctional actin in EEF1A-depleted cells. A partner for EEF1A in cadherin contact maintenance is the formin DIAPH2, which interacts with EEF1A. In contrast, depletion of either the endocytic regulator TRIP10 or the Rho GTPase activator VAV2 reduces E-cadherin levels at junctions. TRIP10 binds to and requires VAV2 function for its junctional localization. Overall, we present new conceptual insights on junction stabilization, which integrate known and novel pathways with impact for epithelial morphogenesis, homeostasis and diseases. 1 National Heart and Lung Institute, Faculty of Medicine, Imperial College London, London SW7 2AZ, UK. 2 Computing Department, Imperial College London, London SW7 2AZ, UK. 3 Bioengineering Department, Faculty of Engineering, Imperial College London, London SW7 2AZ, UK. 4 Department of Surgery & Cancer, Faculty of Medicine, Imperial College London, London SW7 2AZ, UK. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Extraction of Dynamic Patterns from Static Rna Expression Data: an Application to Hematological Neoplasms
EXTRACTION OF DYNAMIC PATTERNS FROM STATIC RNA EXPRESSION DATA: AN APPLICATION TO HEMATOLOGICAL NEOPLASMS Relatore: Barbara Di Camillo Correlatore: Dott.ssa Alessandra Trojani Laureando: Giulia Bianchi ANNO ACCADEMICO 2012/2013 Nothing worth gaining was ever gained without effort. Theodore Roosevelt Index Section 1 INTRODUCTION 1 1.1. Extraction of temporal dynamics from gene expression data 2 1.2. Chronic Lymphocytic Leukemia 3 1.3. IgM Monoclonal Gammopathy of Undetermined Significance and Waldenstrӧm’s Macroglobulinemia 4 Section 2 DATA 7 2.1. CLL Data set 8 2.2. WM/MGUS Data set 8 Section 3 SAMPLE PROGRESSION DISCOVERY 10 3.1. Methods 10 3.2. SPD step by step 12 3.2.1. Input format 12 3.2.2. Gene filtering 12 3.2.3. Clustering 13 3.2.4. Construct MSTs – Compare modules and MSTs 14 3.2.5. Identify modules similar in terms of progression 16 3.3. Results and discussion 17 Section 4 PARAMETER SETTING 19 4.1. Input configuration 19 4.2. Result evaluation 20 4.3. Conclusions 26 Section 5 APPLICATION TO CHRONIC LYMPHOCYTIC LEUKEMIA 28 5.1. Gene selection 28 5.2. SPD results 29 Section 6 APPLICATION TO WALDENSTRÖM’S MACROGLOBULINEMIA AND IgM MGUS 39 6.1. Gene selection 39 6.2. SPD results 40 Section 7 DISCUSSION 45 Acknowledgements 49 Section 8 REFERENCES 51 Section 9 APPENDIX 55 i Section 1 INTRODUCTION Development and evolution of a disease are dynamic processes that, from a molecular point of view, involve changes in some gene expression levels in the involved organs and cells. A possible approach to study the behavior of such dynamic phenomena is to sample individuals, tissues or other relevant units at subsequent time-points throughout the progression. -

Protein Identities in Evs Isolated from U87-MG GBM Cells As Determined by NG LC-MS/MS
Protein identities in EVs isolated from U87-MG GBM cells as determined by NG LC-MS/MS. No. Accession Description Σ Coverage Σ# Proteins Σ# Unique Peptides Σ# Peptides Σ# PSMs # AAs MW [kDa] calc. pI 1 A8MS94 Putative golgin subfamily A member 2-like protein 5 OS=Homo sapiens PE=5 SV=2 - [GG2L5_HUMAN] 100 1 1 7 88 110 12,03704523 5,681152344 2 P60660 Myosin light polypeptide 6 OS=Homo sapiens GN=MYL6 PE=1 SV=2 - [MYL6_HUMAN] 100 3 5 17 173 151 16,91913397 4,652832031 3 Q6ZYL4 General transcription factor IIH subunit 5 OS=Homo sapiens GN=GTF2H5 PE=1 SV=1 - [TF2H5_HUMAN] 98,59 1 1 4 13 71 8,048185945 4,652832031 4 P60709 Actin, cytoplasmic 1 OS=Homo sapiens GN=ACTB PE=1 SV=1 - [ACTB_HUMAN] 97,6 5 5 35 917 375 41,70973209 5,478027344 5 P13489 Ribonuclease inhibitor OS=Homo sapiens GN=RNH1 PE=1 SV=2 - [RINI_HUMAN] 96,75 1 12 37 173 461 49,94108966 4,817871094 6 P09382 Galectin-1 OS=Homo sapiens GN=LGALS1 PE=1 SV=2 - [LEG1_HUMAN] 96,3 1 7 14 283 135 14,70620005 5,503417969 7 P60174 Triosephosphate isomerase OS=Homo sapiens GN=TPI1 PE=1 SV=3 - [TPIS_HUMAN] 95,1 3 16 25 375 286 30,77169764 5,922363281 8 P04406 Glyceraldehyde-3-phosphate dehydrogenase OS=Homo sapiens GN=GAPDH PE=1 SV=3 - [G3P_HUMAN] 94,63 2 13 31 509 335 36,03039959 8,455566406 9 Q15185 Prostaglandin E synthase 3 OS=Homo sapiens GN=PTGES3 PE=1 SV=1 - [TEBP_HUMAN] 93,13 1 5 12 74 160 18,68541938 4,538574219 10 P09417 Dihydropteridine reductase OS=Homo sapiens GN=QDPR PE=1 SV=2 - [DHPR_HUMAN] 93,03 1 1 17 69 244 25,77302971 7,371582031 11 P01911 HLA class II histocompatibility antigen, -

The Roles of Actin-Binding Domains 1 and 2 in the Calcium-Dependent Regulation of Actin Filament Bundling by Human Plastins
Article The Roles of Actin-Binding Domains 1 and 2 in the Calcium-Dependent Regulation of Actin Filament Bundling by Human Plastins Christopher L. Schwebach 1,2, Richa Agrawal 1, Steffen Lindert 1, Elena Kudryashova 1 and Dmitri S. Kudryashov 1,2 1 - Department of Chemistry and Biochemistry, The Ohio State University, Columbus, OH 43210, USA 2 - Molecular, Cellular, and Developmental Biology Program, The Ohio State University, Columbus, OH 43210, USA Correspondence to Dmitri S. Kudryashov: Department of Chemistry and Biochemistry, The Ohio State University, 484 W 12th Ave, 728 Biosciences Building, Columbus, OH 43210, USA. [email protected] http://dx.doi.org/10.1016/j.jmb.2017.06.021 Edited by James Sellers Abstract The actin cytoskeleton is a complex network controlled by a vast array of intricately regulated actin-binding proteins. Human plastins (PLS1, PLS2, and PLS3) are evolutionary conserved proteins that non-covalently crosslink actin filaments into tight bundles. Through stabilization of such bundles, plastins contribute, in an isoform-specific manner, to the formation of kidney and intestinal microvilli, inner ear stereocilia, immune synapses, endocytic patches, adhesion contacts, and invadosomes of immune and cancer cells. All plastins comprise an N-terminal Ca2+-binding regulatory headpiece domain followed by two actin-binding domains (ABD1 and ABD2). Actin bundling occurs due to simultaneous binding of both ABDs to separate actin filaments. Bundling is negatively regulated by Ca2+, but the mechanism of this inhibition remains unknown. In 2+ this study, we found that the bundling abilities of PLS1 and PLS2 were similarly sensitive to Ca (pCa50 ~6.4), whereas PLS3 was less sensitive (pCa50 ~5.9). -

Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments
Biophysical Journal Volume 68 March 1995 1027-1044 1027 Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments Henk L. Granzier and Thomas C. Irving Department of Veterinary and Comparative Anatomy, Pharmacology, and Physiology, Washington State University, Pullman, Washington 99164·6520 USA ABSTRACT The passive tension-sarcomere length relation of rat cardiac muscle was investigated by studying passive (or not activated) single myocytes and trabeculae. The contribution ofcollagen, titin, microtubules, and intermediate filaments to tension and stiffness was investigated by measuring (1) the effects of KCI/KI extraction on both trabeculae and single myocytes, (2) the effect of trypsin digestion on single myocytes, and (3) the effect of colchicine on single myocytes. It was found that over the working range of sarcomeres in the heart (lengths -1.9-2.2 11m), collagen and titin are the most important contributors to passive tension with titin dominating at the shorter end of the working range and collagen at longer lengths. Microtubules made a modest contribution to passive tension in some cells, but on average their contribution was not significant. Finally, intermediate filaments contribl,!ted about 10%to passive tension oftrabeculae at sarcomere lengths from -1.9to 2.1 11m, and theircontribution dropped to only a few percent at longer lengths. At physiological sarcomere lengths of the heart, cardiac titin developed much higher tensions (>20-fold) than did skeletal muscle titin at comparable lengths. This might be related to the finding that cardiac titin has a molecular mass of 2.5 MDa, 0.3-0.5 MDa smaller than titin of mammalian skeletal muscle, which is predicted to result in a much shorter extensible titin segment in the I-band of cardiac muscle. -

Transiently Structured Head Domains Control Intermediate Filament Assembly
Transiently structured head domains control intermediate filament assembly Xiaoming Zhoua, Yi Lina,1, Masato Katoa,b,c, Eiichiro Morid, Glen Liszczaka, Lillian Sutherlanda, Vasiliy O. Sysoeva, Dylan T. Murraye, Robert Tyckoc, and Steven L. McKnighta,2 aDepartment of Biochemistry, University of Texas Southwestern Medical Center, Dallas, TX 75390; bInstitute for Quantum Life Science, National Institutes for Quantum and Radiological Science and Technology, 263-8555 Chiba, Japan; cLaboratory of Chemical Physics, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892-0520; dDepartment of Future Basic Medicine, Nara Medical University, 840 Shijo-cho, Kashihara, Nara, Japan; and eDepartment of Chemistry, University of California, Davis, CA 95616 Contributed by Steven L. McKnight, January 2, 2021 (sent for review October 30, 2020; reviewed by Lynette Cegelski, Tatyana Polenova, and Natasha Snider) Low complexity (LC) head domains 92 and 108 residues in length are, IF head domains might facilitate filament assembly in a manner respectively, required for assembly of neurofilament light (NFL) and analogous to LC domain function by RNA-binding proteins in the desmin intermediate filaments (IFs). As studied in isolation, these IF assembly of RNA granules. head domains interconvert between states of conformational disor- IFs are defined by centrally located α-helical segments 300 to der and labile, β-strand–enriched polymers. Solid-state NMR (ss-NMR) 350 residues in length. These central, α-helical segments are spectroscopic studies of NFL and desmin head domain polymers re- flanked on either end by head and tail domains thought to be veal spectral patterns consistent with structural order. -

Spectrin and Ankyrin Like Proteins in Spermatids and Spermatozoa of the Hamster and Some Other Mammals Ml Kann, La Pradel, J.-P
Spectrin and ankyrin like proteins in spermatids and spermatozoa of the hamster and some other mammals Ml Kann, La Pradel, J.-P. Fouquet To cite this version: Ml Kann, La Pradel, J.-P. Fouquet. Spectrin and ankyrin like proteins in spermatids and spermatozoa of the hamster and some other mammals. Reproduction Nutrition Development, EDP Sciences, 1993, 33 (1), pp.51-61. hal-00899573 HAL Id: hal-00899573 https://hal.archives-ouvertes.fr/hal-00899573 Submitted on 1 Jan 1993 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Original article Spectrin and ankyrin like proteins in spermatids and spermatozoa of the hamster and some other mammals ML Kann LA Pradel JP Fouquet1 1 UFR Biomédicale, Groupe d’Étude de la Formation et de la Maturation du Gamète mâle, 45 rue des Saints-Pères, 75270 Paris Cedex 06; 2 Institut de Biologie Physico-Chimique, Unité CNRS UA 1112 de Neurologie physico-chimique, 13 rue Pierre et Marie Curie, 75005 Paris, France (Received 7 July 1992; accepted 29 October 1992) Summary ― The presence of spectrin and ankyrin-like proteins was investigated during the differ- entiation and maturation of spermatozoa in mammalian species which have previously been studied for actin and calmodulin. -

Spectrin and Maladaptive Remodeling?
STAT3: a link between CaMKII–βIV-spectrin and maladaptive remodeling? Mohit Hulsurkar, … , Ann P. Quick, Xander H.T. Wehrens J Clin Invest. 2018;128(12):5219-5221. https://doi.org/10.1172/JCI124778. Commentary 2+ βIV-Spectrin, along with ankyrin and Ca /calmodulin-dependent kinase II (CaMKII), has been shown to form local signaling domains at the intercalated disc, while playing a key role in the regulation of Na+ and K+ channels in cardiomyocytes. In this issue of the JCI, Unudurthi et al. show that under chronic pressure overload conditions, CaMKII activation leads to βIV-spectrin degradation, resulting in the release of sequestered STAT3 from the intercalated discs. This in turn leads to dysregulation of STAT3-mediated gene transcription, maladaptive remodeling, fibrosis, and decreased cardiac function. Overall, this study presents interesting findings regarding the role of CaMKII and βIV-spectrin under physiological as well as pathological conditions. Find the latest version: https://jci.me/124778/pdf The Journal of Clinical Investigation COMMENTARY STAT3: a link between CaMKII–βIV-spectrin and maladaptive remodeling? Mohit Hulsurkar,1,2 Ann P. Quick,1,2 and Xander H.T. Wehrens1,2,3,4,5,6 1Cardiovascular Research Institute, 2Department of Molecular Physiology and Biophysics, 3Department of Medicine, 4Department of Pediatrics, 5Department of Neuroscience, and 6Center for Space Medicine, Baylor College of Medicine, Houston, Texas, USA. In addition to their physiological roles, β-spectrins may also contribute to patho- β -Spectrin, along with ankyrin and Ca2+/calmodulin-dependent kinase IV logical changes during pressure over- II (CaMKII), has been shown to form local signaling domains at the load that precipitate detrimental cardiac intercalated disc, while playing a key role in the regulation of Na+ and remodeling. -
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
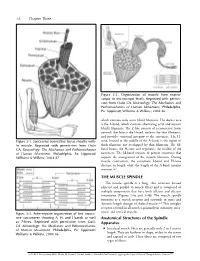
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles.