Finding a Link Between the Hormones of the Somatotropic Axis And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2021 Fur Harvester Digest 3 SEASON DATES and BAG LIMITS
2021 Michigan Fur Harvester Digest RAP (Report All Poaching): Call or Text (800) 292-7800 Michigan.gov/Trapping Table of Contents Furbearer Management ...................................................................3 Season Dates and Bag Limits ..........................................................4 License Types and Fees ....................................................................6 License Types and Fees by Age .......................................................6 Purchasing a License .......................................................................6 Apprentice & Youth Hunting .............................................................9 Fur Harvester License .....................................................................10 Kill Tags, Registration, and Incidental Catch .................................11 When and Where to Hunt/Trap ...................................................... 14 Hunting Hours and Zone Boundaries .............................................14 Hunting and Trapping on Public Land ............................................18 Safety Zones, Right-of-Ways, Waterways .......................................20 Hunting and Trapping on Private Land ...........................................20 Equipment and Fur Harvester Rules ............................................. 21 Use of Bait When Hunting and Trapping ........................................21 Hunting with Dogs ...........................................................................21 Equipment Regulations ...................................................................22 -

Giant Panda Facts (Ailuropoda Melanoleuca)
U.S. Fish & Wildlife Service Giant Panda Facts (Ailuropoda melanoleuca) Giant panda. John J. Mosesso What animal is black and white Giant pandas are bears with one or two cubs weighing 3 to 5 and loved all over the world? If you striking black and white markings. ounces each is born in a sheltered guessed the giant panda, you’re The ears, eye patches, legs and den. Usually only one cub survives. right! shoulder band are black; the rest The eyes open at 1 1/2 to 2 months of the body is whitish. They have and the cub becomes mobile at The giant panda is also known as thick, woolly coats to insulate them approximately three months of the panda bear, bamboo bear, or in from the cold. Adults are four to six age. At 12 months the cub becomes Chinese as Daxiongmao, the “large feet long and may weigh up to 350 totally independent. While their bear cat.” In fact, its scientific pounds—about the same size as average life span in the wild is name means “black and white cat- the American black bear. However, about 15 years, giant pandas in footed animal.” unlike the black bear, giant pandas captivity have been known to live do not hibernate and cannot walk well into their twenties. Giant pandas are found only in on their hind legs. the mountains of central China— Scientists have debated for more in small isolated areas of the The giant panda has unique front than a century whether giant north and central portions of the paws—one of the wrist bones is pandas belong to the bear family, Sichuan Province, in the mountains enlarged and elongated and is used the raccoon family, or a separate bordering the southernmost part of like a thumb, enabling the giant family of their own. -
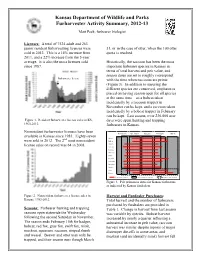
2012-13 Furharvester Activity Summary
Kansas Department of Wildlife and Parks Furharvester Activity Summary, 2012-13 Matt Peek, furbearer biologist Licenses: A total of 7524 adult and 263 junior resident furharvesting licenses were 31, or in the case of otter, when the 100 otter sold in 2012. This is a 14% increase from quota is reached. 2011, and a 22% increase from the 5-year average. It is also the most licenses sold Historically, the raccoon has been the most since 1987. important furbearer species in Kansas in terms of total harvest and pelt value, and season dates are set to roughly correspond Furharvester license with the time when raccoons are prime (Figure 3). In addition to ensuring the different species are conserved, emphasis is placed on having season open for all species at the same time – so a bobcat taken incidentally by a raccoon trapper in November can be kept, and a raccoon taken incidentally by a bobcat trapper in February can be kept. Last season, over 236,000 user Figure 1. Resident furharvester license sales in KS, days were spent hunting and trapping 1960-2012. furbearers in Kansas. Nonresident furharvester licenses have been November December January February March available in Kansas since 1983. Eighty-seven nd Badger were sold in 2012. The 2 most nonresident Bobcat Coyote license sales on record was 64 in 2008. Gray fox Red fox Swift fox Mink Muskrat Opossum Raccoon Skunk Otter Beaver Primeness Period Furharvesting Season Beaver/Otter Season Figure 3. Pelt primeness dates for Kansas furbearers as indicated by Kansas furdealers. Figure 2. Nonresident furharvester license sales in Harvest and Furdealer Purchases: Kansas, 1983-2012. -

OREGON FURBEARER TRAPPING and HUNTING REGULATIONS
OREGON FURBEARER TRAPPING and HUNTING REGULATIONS July 1, 2020 through June 30, 2022 Please Note: Major changes are underlined throughout this synopsis. License Requirements Trapper Education Requirement By action of the 1985 Oregon Legislature, all trappers born after June 30, Juveniles younger than 12 years of age are not required to purchase a 1968, and all first-time Oregon trappers of any age are required to license, except to hunt or trap bobcat and river otter. However, they must complete an approved trapper education course. register to receive a brand number through the Salem ODFW office. To trap bobcat or river otter, juveniles must complete the trapper education The study guide may be completed at home. Testing will take place at course. Juveniles 17 and younger must have completed hunter education Oregon Department of Fish and Wildlife (ODFW) offices throughout the to obtain a furtaker’s license. state. A furtaker’s license will be issued by the Salem ODFW Headquarters office after the test has been successfully completed and Landowners must obtain either a furtaker’s license, a hunting license for mailed to Salem headquarters, and the license application with payment furbearers, or a free license to take furbearers on land they own and on has been received. Course materials are available by writing or which they reside. To receive the free license and brand number, the telephoning Oregon Department of Fish and Wildlife, I&E Division, 4034 landowner must obtain from the Salem ODFW Headquarters office, a Fairview Industrial Drive SE, Salem, OR 97302, (800) 720-6339 x76002. receipt of registration for the location of such land prior to hunting or trapping furbearing mammals on that land. -

GAIT PATTERNS Pacer Diagonal Walker Bounder Galloper RABBITS
GAIT PATTERNS RODENTS Shows - 4 toes front, 5 toes rear, claws General Shape Normal Pace Gait: Galloper Pacer Diagonal Walker Indirect Register Gallopers: Squirrels, Ground Squirrels, Mice Rats, Bounder Chipmunks, Ground Hog, Marmot. Cross Pattern Tree dwellers show both pairs of feet parallel. Ground dwellers show dominant foot landing first. Squirrel Pacers: Porcupine, Muskrat, Beaver, Mountain Beaver Galloper Porcupine, Muskrat, Beaver - in deep mud show 5 toes in front (a hidden thumb). Mountain Beaver - always shoes 5 toes in front. RABBITS & HARES Shows - 4 toes front, 4 toes rear CAT FAMILY Shows - 4 toes front, 4 toes rear, claws (rarely) General Shape Normal Pace Gait: Galloper General Shape Normal Pace Gait: Diagonal Walker Rear Indirect Register Direct Register Elbow on the rear foot may or may not show. Front feet 1/2 larger than rear No claws (95% of time) - sometimes out during a hunt. Rabbit - rear feet 2 times larger than front feet Round Zero straddle Hare - rear feet 4-5 times larger than front. Zero pitch Front Rear The small heel pad helps to distinguish between a showshoe hare with no elbow showing and a Feral Cat - 4 toes equal size with elbow dog galloping Rabbit Mountain Lion - 4 toes equal size Cat Bobcat - inner toes larger, cleft in heel pad Lynx - outer toes larger DOG FAMILY Shows - 4 toes front, 4 toes rear, claws WEASEL FAMILY Shows - 5 toes front, 5 toes rear, claws General Shape Normal Pace Gait: Diagonal Walker General Shape Normal Pace Gait: Bounder Indirect Register Indirect Register Front feet 1/3 larger than rear. -

GRAY FOX Urocyon Cinereoargenteus
WILDLIFE IN CONNECTICUT INFORMATIONAL SERIES GRAY FOX Urocyon cinereoargenteus Habitat: Deciduous woodlands, thickets and swampy Length: 32 to 45 inches. Sexes about equal in size. areas. Food: Rabbits, mice, voles, rabbits, chipmunks, Weight: Ranges from 7 to 14 pounds, 10 to 11 pounds squirrels, fruits, insects, birds and eggs, carrion, corn, is average. amphibians and reptiles. Identification: Foxes have pointed ears, an elongated under out-buildings such as barns and sheds. Most snout (shorter and more cat-like in appearance in the foxes have more than one den and will readily move gray fox than the red fox) and a long, bushy tail which is their young if disturbed. The pups stay in the den until carried horizontally. The gray fox is somewhat stout about four to five weeks of age, after which they emerge and has shorter legs than the red fox. Its coat is mostly and begin to play outside the den entrance. Both adults grizzled-gray. The sides of the neck, back of the ears, a care for the young by bringing food and guarding the band across the chest, the inner and back surfaces of den site. At about 12 weeks of age, the pups are the legs, the feet, the sides of the belly and the under weaned and join the adults on hunting forays, learning to surface of the tail are all reddish-brown. The cheeks, catch food for themselves. In the fall, the young dis- throat, inner ears and most of the underside are white. perse from the family unit and will usually breed the first The upper part of the tail, including the tip, is black. -

Individual Identification of Raccoons (Procyon Lotor) Using Track Plate Foot Printing Author(S): Stephanie A
Individual Identification of Raccoons (Procyon lotor) Using Track Plate Foot Printing Author(s): Stephanie A. Ellison and Bradley J. Swanson Source: The American Midland Naturalist, 176(2):306-312. Published By: University of Notre Dame DOI: http://dx.doi.org/10.1674/0003-0031-176.2.306 URL: http://www.bioone.org/doi/full/10.1674/0003-0031-176.2.306 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Am. Midl. Nat. (2016) 176:306–312 Notes and Discussion Piece Individual Identification of Raccoons (Procyon lotor) Using Track Plate Foot Printing ABSTRACT.—Population studies are widely used in conservation and management efforts, but acquiring necessary data sets can be difficult. Convenience sampling or camera monitoring may result in biased outcomes, while explicit approaches such as genetic analysis may be impractical due to cost and time. -

Download Vol. 39, No. 6
... r , 5 Mt; , - J.$.I' ~''i. I I I of the FLORIDA MUSEUM OF NATURAL HISTORY BODY MASS AND SKULL MEASUREMENTS IN FOUR JAGUAR POPULATIONS AND OBSERVATIONS ON THEIR PREY BASE Rafael Hoogesteijn and Edgardo Mondolfi Volume 39, No. 6 pp. 195-219 1996 1 - 'Ii;*5' 3'-*t-lf-' I + ' ''. ' '·*'*114/I.M.' "' t Jit:j *40 k 2 JE <111111Pip rEL- fi;7~AilhRE'F .1 1 d.- 11 4 -A-- / _I_ r It 5 T *43 MI 5* -:IA UNIVERSITY OF FLORIDA GAINESVILLE Numbers of the BULLETIN OF THE FLORIDA MUSEUM OF NATURAL HISTORY am published at irregular intervals. Volumes contain about 300 pages and are not necessarily completed in any one calendar year. JOHN F. EISENBERG, EDITOR RICHARD FRANZ, CO-EDITOR RHODA J. BRYANT, MANAGING EDrrOR Communications concerning purchase or exchange of the publications and all manuscripts should be addressed to: Managing Editor, Bulletin; Florida Museum of Natural History; University of Florida; P. O. Box 117800, Gainesville FL 32611-7800; U.S.A This journal is printed on recycled paper. ISSN: 0071-6154 CODEN: BF 5BA5 Publication date: September 30,1996 Price: $1.35 BODY MASS AND SKULL MEASUREMENTS IN FOUR JAGUAR POPULATIONS AND OBSERVATIONS ON THEIR PREY BASE Rafael Hoogesteijnt and Edgardo Mondollf ABSTRACT Body mass and nine skull measurements of two floodplain (Pantanal and Llanos) and two forest (Amazon and Central America) jaguar (Panthem onca) populations, were analyzed to compare them, relate their morphometric dimensions to preybase and latitude, and examine the relationship with their subspecies status. Analyzing data from males and females separately, jaguar at all sites differed significantly for most variables studied, with the exception of rostral breadth, maxillary teeth row length, and pterygoid fossa breadth for both sexes, and postorbital breadth for females, which were either not or only weakly significant. -

LARGE CANID (Canidae) CARE MANUAL
LARGE CANID (Canidae) CARE MANUAL CREATED BY THE AZA Canid Taxon Advisory Group IN ASSOCIATION WITH THE AZA Animal Welfare Committee Large Canid (Canidae) Care Manual Large Canid (Canidae) Care Manual Published by the Association of Zoos and Aquariums in association with the AZA Animal Welfare Committee Formal Citation: AZA Canid TAG 2012. Large Canid (Canidae) Care Manual. Association of Zoos and Aquariums, Silver Spring, MD. p.138. Authors and Significant contributors: Melissa Rodden, Smithsonian Conservation Biology Institute, AZA Maned Wolf SSP Coordinator. Peter Siminski, The Living Desert, AZA Mexican Wolf SSP Coordinator. Will Waddell, Point Defiance Zoo and Aquarium, AZA Red Wolf SSP Coordinator. Michael Quick, Sedgwick County Zoo, AZA African Wild Dog SSP Coordinator. Reviewers: Melissa Rodden, Smithsonian Conservation Biology Institute, AZA Maned Wolf SSP Coordinator. Peter Siminski, The Living Desert, AZA Mexican Wolf SSP Coordinator. Will Waddell, Point Defiance Zoo and Aquarium, AZA Red Wolf SSP Coordinator. Michael Quick, Sedgwick County Zoo, AZA African Wild Dog SSP Coordinator. Mike Maslanka, Smithsonian’s National Zoo, AZA Nutrition Advisory Group Barbara Henry, Cincinnati Zoo & Botanical Garden, AZA Nutrition Advisory Group Raymond Van Der Meer, DierenPark Amersfoort, EAZA Canid TAG Chair. Dr. Michael B. Briggs, DVM, MS, African Predator Conservation Research Organization, CEO/Principle Investigator. AZA Staff Editors: Katie Zdilla, B.A. AZA Conservation and Science Intern Elisa Caballero, B.A. AZA Conservation and Science Intern Candice Dorsey, Ph.D. AZA Director, Animal Conservation Large Canid Care Manual project consultant: Joseph C.E. Barber, Ph.D. Cover Photo Credits: Brad McPhee, red wolf Bert Buxbaum, African wild dog and Mexican gray wolf Lisa Ware, maned wolf Disclaimer: This manual presents a compilation of knowledge provided by recognized animal experts based on the current science, practice, and technology of animal management. -

Emammal Animal Identification Guide
Animal Identification Guide Distinctive Species: White Tailed Deer White tail deer have a tan to reddish-brown coat in the summer and slightly duller color variations during the winter. Males possess antlers during the summer months which are shed during the winter. As the name suggests, white tailed deer have brown tails with a white underside, and often white underbellies. Fawns have reddish coats, and while they are still young have white spots along their backs and sides. They are common in forests; especially ones that have open fields or brush lands, as they feed predominantly on grasses and other vegetation. White-tailed deer are found almost anywhere in the United States. Northern Raccoon Northern raccoons are common almost anywhere in the United States. Their most obvious features are their ringed tails, white faces, and mask-like patches around the eyes. Northern raccoons have coarse looking fur that usually ranges from black to gray, although brown, red and albino raccoons have also been documented. They are well known as being scavengers, and therefore can live in almost any environment that has water and some sort of shelter. They are extremely curious animals and close-up pictures of raccoon faces are common on camera-traps. Virginia Opossum Virginia opossums, a predominantly nocturnal scavenging species native to the southern United States, sport a white head and predominant long, furless pink tail. These opossums have scruffy looking gray body fur, as well as small, leathery ears and a pointed, pink snout. Virginia opossums are often found in forests and woodlands, but due to their scavenging nature are also found in urban areas as well. -

Texas Wildlife Identification Guide
TEXAS WILDLIFE IDENTIFICATION GUIDE A guide to game animals, game birds, furbearers and other wildlife of Texas. INTRODUCTION Texas game animals, game birds, furbearers and other wildlife are important for many reasons. They provide countless hours of viewing and recreational opportunities. They benefit the Texas economy through hunting and “nature tourism” such as birdwatching. Commercial businesses that provide birdseed, dry corn and native landscaping may be devoted solely to attracting many of the animals found in this book. Local hunting and trapping economies, guiding operations and hunting leases have prospered because of the abun- dance of these animals in Texas. The Texas Parks and Wildlife Department benefits because of hunting license sales, but it uses these funds to research, manage and protect all wildlife populations – not just game animals. Game animals provide humans with cultural, social, aesthetic and spiritual pleasures found in wildlife art, taxidermy and historical artifacts. Conservation organiza- tions dedicated to individual species such as quail, turkey and deer, have funded thousands of wildlife projects throughout North America, demonstrating the mystique game animals have on people. Animals referenced in this pocket guide exist because their habitat exists in Texas. Habitat is food, cover, water and space, all suitably arranged. They are part of a vast food chain or web that includes thousands more species of wildlife such as the insects, non-game animals, fish and i rare/endangered species. Active management of wild landscapes is the primary means to continue having abundant populations of wildlife in Texas. Preservation of rare and endangered habitat is one way of saving some species of wildlife such as the migratory whooping crane that makes Texas its home in the winter. -

Hall of North American Mammals Educator's Guide
Educator’s Guide THE JILL AND LEWIS BERNARD FAMILY HALL OF NORTH AMERICAN MAMMALS Featuring Carnivorans INSIDE: • Suggestions to Help You Come Prepared • Essential Questions for Student Inquiry • Strategies for Teaching in the Exhibition • Map of the Exhibition • Online Resources for the Classroom • Correlation to Standards • Glossary amnh.org/namammals Essential QUESTIONS More than 25 Museum expeditions across this continent produced the specimens displayed in this hall’s magnificent dioramas. Many belong to the order of mammals called Carnivora (carnivorans), one of the most diverse orders within the mammal group. Use the Essential Questions below to connect the dioramas to your curriculum. What is a mammal? How have traits of the Carnivora helped You might have grown up thinking that all mammals share the order survive and diversify? certain traits, like fur and giving birth to live young, and As environments change over time, living things must most living mammals do. But what defines this diverse respond by migrating, adapting, or sometimes going group of animals is that they all are descended from a com- extinct. Different traits are favored in different habitats mon ancestor shared with no other living animals. Because and are passed on to future generations. For example, of this common ancestor (and unlike other vertebrates), carnivorans take care of their young until they are old all mammals have three middle ear bones. The group is enough to hunt, which helps them live to adulthood. divided into over 20 orders, which include Primates (e.g. Also, the diversity of their teeth has helped carnivorans humans), Rodentia (e.g.