Download Vol. 39, No. 6
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Baylisascariasis
Baylisascariasis Importance Baylisascaris procyonis, an intestinal nematode of raccoons, can cause severe neurological and ocular signs when its larvae migrate in humans, other mammals and birds. Although clinical cases seem to be rare in people, most reported cases have been Last Updated: December 2013 serious and difficult to treat. Severe disease has also been reported in other mammals and birds. Other species of Baylisascaris, particularly B. melis of European badgers and B. columnaris of skunks, can also cause neural and ocular larva migrans in animals, and are potential human pathogens. Etiology Baylisascariasis is caused by intestinal nematodes (family Ascarididae) in the genus Baylisascaris. The three most pathogenic species are Baylisascaris procyonis, B. melis and B. columnaris. The larvae of these three species can cause extensive damage in intermediate/paratenic hosts: they migrate extensively, continue to grow considerably within these hosts, and sometimes invade the CNS or the eye. Their larvae are very similar in appearance, which can make it very difficult to identify the causative agent in some clinical cases. Other species of Baylisascaris including B. transfuga, B. devos, B. schroeder and B. tasmaniensis may also cause larva migrans. In general, the latter organisms are smaller and tend to invade the muscles, intestines and mesentery; however, B. transfuga has been shown to cause ocular and neural larva migrans in some animals. Species Affected Raccoons (Procyon lotor) are usually the definitive hosts for B. procyonis. Other species known to serve as definitive hosts include dogs (which can be both definitive and intermediate hosts) and kinkajous. Coatimundis and ringtails, which are closely related to kinkajous, might also be able to harbor B. -

Pleistocene Mammals and Paleoecology of the Western Amazon
PLEISTOCENE MAMMALS AND PALEOECOLOGY OF THE WESTERN AMAZON By ALCEU RANCY A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 1991 . To Cleusa, Bianca, Tiago, Thomas, and Nono Saul (Pistolin de Oro) . ACKNOWLEDGMENTS This work received strong support from John Eisenberg (chairman) and David Webb, both naturalists, humanists, and educators. Both were of special value, contributing more than the normal duties as members of my committee. Bruce MacFadden provided valuable insights at several periods of uncertainty. Ronald Labisky and Kent Redford also provided support and encouragement. My field work in the western Amazon was supported by several grants from the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq) , and the Universidade Federal do Acre (UFAC) , Brazil. I also benefitted from grants awarded to Ken Campbell and Carl Frailey from the National Science Foundation (NSF) I thank Daryl Paul Domning, Jean Bocquentin Villanueva, Jonas Pereira de Souza Filho, Ken Campbell, Jose Carlos Rodrigues dos Santos, David Webb, Jorge Ferigolo, Carl Frailey, Ernesto Lavina, Michael Stokes, Marcondes Costa, and Ricardo Negri for sharing with me fruitful and adventurous field trips along the Amazonian rivers. The CNPq and the Universidade Federal do Acre, supported my visit to the. following institutions (and colleagues) to examine their vertebrate collections: iii . ; ; Universidade do Amazonas, Manaus -

Phylogeographic and Diversification Patterns of the White-Nosed Coati
Molecular Phylogenetics and Evolution 131 (2019) 149–163 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Phylogeographic and diversification patterns of the white-nosed coati (Nasua narica): Evidence for south-to-north colonization of North America T ⁎ Sergio F. Nigenda-Moralesa, , Matthew E. Gompperb, David Valenzuela-Galvánc, Anna R. Layd, Karen M. Kapheime, Christine Hassf, Susan D. Booth-Binczikg, Gerald A. Binczikh, Ben T. Hirschi, Maureen McColginj, John L. Koprowskik, Katherine McFaddenl,1, Robert K. Waynea, ⁎ Klaus-Peter Koepflim,n, a Department of Ecology & Evolutionary Biology, University of California, Los Angeles, Los Angeles, CA 90095, USA b School of Natural Resources, University of Missouri, Columbia, MO 65211, USA c Departamento de Ecología Evolutiva, Centro de Investigación en Biodiversidad y Conservación, Universidad Autónoma del Estado de Morelos, Cuernavaca, Morelos 62209, Mexico d Department of Pathology and Laboratory Medicine, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA 90095, USA e Department of Biology, Utah State University, Logan, UT 84322, USA f Wild Mountain Echoes, Vail, AZ 85641, USA g New York State Department of Environmental Conservation, Albany, NY 12233, USA h Amsterdam, New York 12010, USA i Zoology and Ecology, College of Science and Engineering, James Cook University, Townsville, QLD 4811, Australia j Department of Biological Sciences, Purdue University, West Lafayette, IN 47907, USA k School of Natural Resources and the Environment, The University of Arizona, Tucson, AZ 85721, USA l College of Agriculture, Forestry and Life Sciences, Clemson University, Clemson, SC 29634, USA m Smithsonian Conservation Biology Institute, National Zoological Park, Washington, D.C. -

Activity Pattern of Medium and Large Sized Mammals and Density Estimates of Cuniculus Paca (Rodentia: Cuniculidae) in the Brazilian Pampa C
Brazilian Journal of Biology http://dx.doi.org/10.1590/1519-6984.174403 ISSN 1519-6984 (Print) Original Article ISSN 1678-4375 (Online) Activity pattern of medium and large sized mammals and density estimates of Cuniculus paca (Rodentia: Cuniculidae) in the Brazilian Pampa C. Leuchtenbergera*, Ê. S. de Oliveirab, L. P. Cariolattoc and C. B. Kasperb aInstituto Federal Farroupilha – IFFAR, Coordenação de Ciências Biológicas, Rua Erechim, nº 860, Campus Panambi, CEP 98280-000, Panambi, RS, Brazil bUniversidade Federal do Pampa – UNIPAMPA, Laboratório de Biologia de Mamíferos e Aves – LABIMAVE, Av. Antônio Trilha, nº 1847, CEP 97300-000, São Gabriel, RS, Brazil cInstituto Federal Farroupilha – IFFAR, RS-377, Km 27, Campus Alegrete, Passo Novo, CEP 97555-000, Alegrete, RS, Brazil *e-mail: [email protected] Received: January 13, 2017 – Accepted: June 26, 2017 – Distributed: November 30, 2018 (With 2 figures) Abstract Between July 2014 and April 2015, we conducted weekly inventories of the circadian activity patterns of mammals in Passo Novo locality, municipality of Alegrete, southern Brazil. The vegetation is comprised by a grassy-woody steppe (grassland). We used two camera traps alternately located on one of four 1 km transects, each separated by 1 km. We classified the activity pattern of species by the percentage of photographic records taken in each daily period. We identify Cuniculus paca individuals by differences in the patterns of flank spots. We then estimate the density 1) considering the area of riparian forest present in the sampling area, and 2) through capture/recapture analysis. Cuniculus paca, Conepatus chinga and Hydrochoerus hydrochaeris were nocturnal, Cerdocyon thous had a crepuscular/nocturnal pattern, while Mazama gouazoubira was cathemeral. -

2021 Fur Harvester Digest 3 SEASON DATES and BAG LIMITS
2021 Michigan Fur Harvester Digest RAP (Report All Poaching): Call or Text (800) 292-7800 Michigan.gov/Trapping Table of Contents Furbearer Management ...................................................................3 Season Dates and Bag Limits ..........................................................4 License Types and Fees ....................................................................6 License Types and Fees by Age .......................................................6 Purchasing a License .......................................................................6 Apprentice & Youth Hunting .............................................................9 Fur Harvester License .....................................................................10 Kill Tags, Registration, and Incidental Catch .................................11 When and Where to Hunt/Trap ...................................................... 14 Hunting Hours and Zone Boundaries .............................................14 Hunting and Trapping on Public Land ............................................18 Safety Zones, Right-of-Ways, Waterways .......................................20 Hunting and Trapping on Private Land ...........................................20 Equipment and Fur Harvester Rules ............................................. 21 Use of Bait When Hunting and Trapping ........................................21 Hunting with Dogs ...........................................................................21 Equipment Regulations ...................................................................22 -

Mammalia, Carnivora) from the Blancan of Florida
THREE NEW PROCYONIDS (MAMMALIA, CARNIVORA) FROM THE BLANCAN OF FLORIDA Laura G. Emmert1,2 and Rachel A. Short1,3 ABSTRACT Fossils of the mammalian family Procyonidae are relatively abundant at many fossil localities in Florida. Analysis of specimens from 16 late Blancan localities from peninsular Florida demonstrate the presence of two species of Procyon and one species of Nasua. Procyon gipsoni sp. nov. is slightly larger than extant Procyon lotor and is distinguished by five dental characters including a lack of a crista between the para- cone and hypocone on the P4, absence of a basin at the lingual intersection of the hypocone and protocone on the P4, and a reduced metaconule on the M1. Procyon megalokolos sp. nov. is significantly larger than extant P. lotor and is characterized primarily by morphology of the postcrania, such as an expanded and posteriorly rotated humeral medial epicondyle, more prominent tibial tuberosity, and more pronounced radioulnar notch. Other than larger size, the dentition of P. megalokolos falls within the range of variation observed in extant P. lotor, suggesting that it may be an early member of the P. lotor lineage. Nasua mast- odonta sp. nov. has a unique accessory cusp on the m1 as well as multiple morphological differences in the dentition and postcrania, such as close appression of the trigonid of the m1 and a less expanded medial epicondyle of the humerus. We also synonymize Procyon rexroadensis, formerly the only known Blancan Procyon species in North America, with P. lotor due to a lack of distinct dental morphological features observed in specimens from its type locality in Kansas. -

Mammalian Predators Appropriating the Refugia of Their Prey
Mamm Res (2015) 60:285–292 DOI 10.1007/s13364-015-0236-y ORIGINAL PAPER When prey provide more than food: mammalian predators appropriating the refugia of their prey William J. Zielinski 1 Received: 30 September 2014 /Accepted: 20 July 2015 /Published online: 31 July 2015 # Mammal Research Institute, Polish Academy of Sciences, Białowieża, Poland (outside the USA) 2015 Abstract Some mammalian predators acquire both food and predators) may play disproportionately important roles in their shelter from their prey, by eating them and using the refugia communities. the prey construct. I searched the literature for examples of predators that exhibit this behavior and summarize their taxo- Keywords Predator–prey . Dens . Herbivore . Behavior . nomic affiliations, relative sizes, and distributions. I hypothe- Habitat . Resting . Foraging sized that size ratios of species involved in this dynamic would be near 1.0, and that most of these interactions would occur at intermediate and high latitudes. Seventeen species of Introduction Carnivorans exploited at least 23 species of herbivores as food and for their refugia. Most of them (76.4 %) were in the Mammals require food and most require shelter, either to pro- Mustelidae; several small species of canids and a few tect them from predators or from thermal stress. Carnivorous herpestids were exceptions. Surprisingly, the average mammals are unique in that they subsist on mobile food predator/prey weight ratio was 10.51, but few species of pred- sources which, particularly if these sources are vertebrates, ators were more than ten times the weight of the prey whose may build their own refuges to help regulate their body tem- refugia they exploit. -

An Educator's Resource to Texas Mammal Skulls and Skins
E4H-014 11/17 An Educator’s Resource to Texas Mammal Skulls and Skins for use in 4-H Wildlife Programs and FFA Wildlife Career Development Events By, Denise Harmel-Garza Program Coordinator I, Texas A&M AgriLife Extension Service, 4-H Photographer and coauthor, Audrey Sepulveda M.Ed. Agricultural Leadership, Education and Communications, Texas A&M University College Station, Texas 2017 “A special thanks to the Biodiversity Research and Teaching Collections at Texas A&M University for providing access to their specimens.” Texas A&M AgriLife Extension provides equal opportunities in its programs and employment to all persons, regardless of race, color, sex, religion, national origin, disability, age, genetic information, veteran status, sexual orientation, or gender identity. The Texas A&M University System, U.S. Department of Agriculture, and the County Commissioners Courts of Texas Cooperating. Introduction Texas youth that participate in wildlife programs may be asked to identify a skull, skin, scat, tracks, etc. of an animal. Usually, educators must find this information and assemble pictures of skulls and skins from various sources. They also must ensure that what they find is relevant and accurate. Buying skulls and skins to represent all Texas mammals is costly. Most educators cannot afford them, and if they can, maintaining these collections over time is problematic. This study resource will reduce the time teachers across the state need to spend searching for information and allow them more time for presenting the material to their students. This identification guide gives teachers and students easy access to information that is accurate and valuable for learning to identify Texas mammals. -

Museum of Natural History
p m r- r-' ME FYF-11 - - T r r.- 1. 4,6*. of the FLORIDA MUSEUM OF NATURAL HISTORY THE COMPARATIVE ECOLOGY OF BOBCAT, BLACK BEAR, AND FLORIDA PANTHER IN SOUTH FLORIDA David Steffen Maehr Volume 40, No. 1, pf 1-176 1997 == 46 1ms 34 i " 4 '· 0?1~ I. Al' Ai: *'%, R' I.' I / Em/-.Ail-%- .1/9" . -_____- UNIVERSITY OF FLORIDA GAINESVILLE Numbers of the BULLETIN OF THE FLORIDA MUSEUM OF NATURAL HISTORY am published at irregular intervals Volumes contain about 300 pages and are not necessarily completed in any one calendar year. JOHN F. EISENBERG, EDITOR RICHARD FRANZ CO-EDIWR RHODA J. BRYANT, A£ANAGING EMOR Communications concerning purchase or exchange of the publications and all manuscripts should be addressed to: Managing Editor. Bulletin; Florida Museum of Natural Histoty, University of Florida P. O. Box 117800, Gainesville FL 32611-7800; US.A This journal is printed on recycled paper. ISSN: 0071-6154 CODEN: BF 5BAS Publication date: October 1, 1997 Price: $ 10.00 Frontispiece: Female Florida panther #32 treed by hounds in a laurel oak at the site of her first capture on the Florida Panther National Wildlife Refuge in central Collier County, 3 February 1989. Photograph by David S. Maehr. THE COMPARATIVE ECOLOGY OF BOBCAT, BLACK BEAR, AND FLORIDA PANTHER IN SOUTH FLORIDA David Steffen Maehri ABSTRACT Comparisons of food habits, habitat use, and movements revealed a low probability for competitive interactions among bobcat (Lynx ndia). Florida panther (Puma concotor cooi 1 and black bear (Urns amencanus) in South Florida. All three species preferred upland forests but ©onsumed different foods and utilized the landscape in ways that resulted in ecological separation. -

University of Florida Thesis Or Dissertation Formatting
UNDERSTANDING CARNIVORAN ECOMORPHOLOGY THROUGH DEEP TIME, WITH A CASE STUDY DURING THE CAT-GAP OF FLORIDA By SHARON ELIZABETH HOLTE A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2018 © 2018 Sharon Elizabeth Holte To Dr. Larry, thank you ACKNOWLEDGMENTS I would like to thank my family for encouraging me to pursue my interests. They have always believed in me and never doubted that I would reach my goals. I am eternally grateful to my mentors, Dr. Jim Mead and the late Dr. Larry Agenbroad, who have shaped me as a paleontologist and have provided me to the strength and knowledge to continue to grow as a scientist. I would like to thank my colleagues from the Florida Museum of Natural History who provided insight and open discussion on my research. In particular, I would like to thank Dr. Aldo Rincon for his help in researching procyonids. I am so grateful to Dr. Anne-Claire Fabre; without her understanding of R and knowledge of 3D morphometrics this project would have been an immense struggle. I would also to thank Rachel Short for the late-night work sessions and discussions. I am extremely grateful to my advisor Dr. David Steadman for his comments, feedback, and guidance through my time here at the University of Florida. I also thank my committee, Dr. Bruce MacFadden, Dr. Jon Bloch, Dr. Elizabeth Screaton, for their feedback and encouragement. I am grateful to the geosciences department at East Tennessee State University, the American Museum of Natural History, and the Museum of Comparative Zoology at Harvard for the loans of specimens. -

Giant Panda Facts (Ailuropoda Melanoleuca)
U.S. Fish & Wildlife Service Giant Panda Facts (Ailuropoda melanoleuca) Giant panda. John J. Mosesso What animal is black and white Giant pandas are bears with one or two cubs weighing 3 to 5 and loved all over the world? If you striking black and white markings. ounces each is born in a sheltered guessed the giant panda, you’re The ears, eye patches, legs and den. Usually only one cub survives. right! shoulder band are black; the rest The eyes open at 1 1/2 to 2 months of the body is whitish. They have and the cub becomes mobile at The giant panda is also known as thick, woolly coats to insulate them approximately three months of the panda bear, bamboo bear, or in from the cold. Adults are four to six age. At 12 months the cub becomes Chinese as Daxiongmao, the “large feet long and may weigh up to 350 totally independent. While their bear cat.” In fact, its scientific pounds—about the same size as average life span in the wild is name means “black and white cat- the American black bear. However, about 15 years, giant pandas in footed animal.” unlike the black bear, giant pandas captivity have been known to live do not hibernate and cannot walk well into their twenties. Giant pandas are found only in on their hind legs. the mountains of central China— Scientists have debated for more in small isolated areas of the The giant panda has unique front than a century whether giant north and central portions of the paws—one of the wrist bones is pandas belong to the bear family, Sichuan Province, in the mountains enlarged and elongated and is used the raccoon family, or a separate bordering the southernmost part of like a thumb, enabling the giant family of their own. -
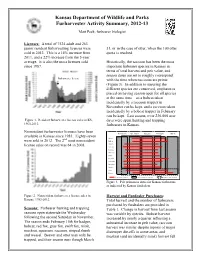
2012-13 Furharvester Activity Summary
Kansas Department of Wildlife and Parks Furharvester Activity Summary, 2012-13 Matt Peek, furbearer biologist Licenses: A total of 7524 adult and 263 junior resident furharvesting licenses were 31, or in the case of otter, when the 100 otter sold in 2012. This is a 14% increase from quota is reached. 2011, and a 22% increase from the 5-year average. It is also the most licenses sold Historically, the raccoon has been the most since 1987. important furbearer species in Kansas in terms of total harvest and pelt value, and season dates are set to roughly correspond Furharvester license with the time when raccoons are prime (Figure 3). In addition to ensuring the different species are conserved, emphasis is placed on having season open for all species at the same time – so a bobcat taken incidentally by a raccoon trapper in November can be kept, and a raccoon taken incidentally by a bobcat trapper in February can be kept. Last season, over 236,000 user Figure 1. Resident furharvester license sales in KS, days were spent hunting and trapping 1960-2012. furbearers in Kansas. Nonresident furharvester licenses have been November December January February March available in Kansas since 1983. Eighty-seven nd Badger were sold in 2012. The 2 most nonresident Bobcat Coyote license sales on record was 64 in 2008. Gray fox Red fox Swift fox Mink Muskrat Opossum Raccoon Skunk Otter Beaver Primeness Period Furharvesting Season Beaver/Otter Season Figure 3. Pelt primeness dates for Kansas furbearers as indicated by Kansas furdealers. Figure 2. Nonresident furharvester license sales in Harvest and Furdealer Purchases: Kansas, 1983-2012.