Interpretation of Full Blood Count Parameters in Health and Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VIROLOGY for MEDICAL LABORATORY STUDENTS Mwamisi, Muthwii, Mwala, Mokua
VIROLOGY for MEDICAL LABORATORY STUDENTS Mwamisi, Muthwii, Mwala, Mokua To the Reader Virology is an important subject in Medical Laboratory Sciences. Medical Laboratory Medicine puts a technologist/technician in the fore front in diagnosis and proper interpretation of the correct diseases by a clinician. Research on viruses, therefore, has gained a lot of momentum in recent years due to the Medical importance they contribute to the field of medicine. A lot of this work goes through the medical laboratory Department where it is handled by the medical laboratory students, especially, on practical experience. The purpose of this manual is, therefore, to provide to the student adequate up-to-date information in preparation for the work that the student will perform in any Medical Laboratory Department, whether research or routine work. We hope this information will not only be useful to the medical laboratory Students or staff, but!also will be a reference material for others in Health or health related field. Joseph M. Mwamisi, PhD Samson M. Muthwii PhD Denis M. Mwala M.Se. John M. Mokua M.Se., ) 2 TABLE OF CONTENTS 1. VIROLOGY 6 1.1 Definition of Virology 6 1.2 Definition of Viruses 6 1.3 Differences between Bacteria and Viruses 7 1.4 Morphology of Viruses 7 2. CLASSIFICATION OF VIRUSES 9 2.1 Classification 9 Epidemiological Analysis 9 Physical and Chemical Properties Analysis 9 DNA Viruses 9 RNA Viruses 9 Enteric Viruses 10 Respiratory Viruses ' 10 Arthropod-Borne Viruses (Arboviruses) 10 2.2. DNA Viruses 10 Poxvirusae 10 Ridoviridae 10 2.3. RNA Viruses 10 Picornaviridae 11 3. -

Medical Laboratory Scientist LIMITED ENTRY BACHELOR of APPLIED SCIENCE (BAS) REQUIRED CREDITS: 120 DEGREE CODE: MLS-BAS
MEDICAL LABORATORY PROGRAM Medical Laboratory Scientist LIMITED ENTRY BACHELOR OF APPLIED SCIENCE (BAS) REQUIRED CREDITS: 120 DEGREE CODE: MLS-BAS This is a limited-entry program. Some of these courses are program prerequisites and MUST be completed before a student is considered eligible for entry into the Program. Students MUST attend a Health Programs orientation and meet with a Health Programs advisor for additional advisement. DESCRIPTION The Medical Laboratory Scientist (MLS) is an important member of the health care team in hospitals, clinics, medical research and teaching centers, and is an indispensable participant with physicians in providing critical diagnostic information. The MLS functions as a dependable, ambitious and highly motivated professional capable of handling high stress situations with ease and confidence. The Medical Laboratory Scientist performs and interprets diagnostic laboratory procedures using state-of-the-art instrumentation to aid in the detection, diagnosis, and treatment of disease; monitors the standards of accuracy and precision in the performance of tests; performs routine maintenance; analyzes and corrects instrument problems; researches, evaluates, and implements new procedures; and may be responsible for fiscal/personnel management of laboratory. The Bachelor of Applied Science degree in Medical Laboratory Scientist combines academic and laboratory courses on campus with practical experience at clinical affiliate sites. The program is accredited by the National Accrediting Agency for Clinical Laboratory Sciences (NAACLS), 5600 N. River Road, Suite 720, Rosemont, IL 60018-5119, (877) 939-3597. Students successfully completing the program are eligible to take a national certifying examination. STUDENT LEARNING OUTCOMES • Select appropriate courses of action in accordance with established laboratory procedures. -

Medical Laboratory Assistant, a Suggested Guide for A
p .7/ tWI .7- y,1.. P O R T R ESUM ED 013 321 VT 002 2L4 MEDICAL LABORATORY ASSISTANT, A SUGGESTEDGUIDE FOR A TRAINING PROGRAM. OFFICE OF EDUCATION, WASHINGTON, D.C. REPORT NUMBER 0E-87017 PUB DATE 66 EDRS PRICE MF-$0.50 HC-$4.92 123P. DESCRIPTORS-. *MEDICAL LABORATORY ASSISTANTS,TEACHING GUIDES, *PROGRAM PLANNING, PROGRAM DEVELOPMENT,*CURRICULUM GUIDES, CURRICULUM, *HEALTH OCCUPATIONSEDUCATION, POST SECONDARY EDUCATION, MDTA PROGRAMS, INFORMATION IS GIVEN TO ASSIST IN ORGANIZINGAND ADMINISTERING A TRAINING PROGRAM FOR MEDICAL LABORATORY ASSISTANTS IN A VARIETY OF SETTINGS AND TO PROVIDEGUIDANCE IN ESTABLISHING NEW PROGRAMS AND IN EVALUATINGEXISTING ONES. THE MATERIAL WAS PREPARED UNDER THE DIRECTIONOF THE NATIONAL COMMITTEE =OR CAREERS IN MEDICAL TECHNOLOGY.PATHOLOGISTS AND MEDICAL TECHNOLOGISTS PARTICIPATEDIN THE ORGANIZATIONAL AND DEVELOPMENTAL STAGES. ALL MATERIAL WAS REVIEWEDBY A REPRESENTATIVE NATIONAL GROUP OF EXPERTCONSULTANTS IN THE FIELD OF LABORATORY MEDICINE. THE 12-MONTH PROGRAM WAS DESIGNED FOR HIGH SCHOOL GRADUATES OR THEIREQUIVALENT TO SE ADMINISTERED BY A TEACHING STAFF COMPOSEDOF A NATIONAL DIRECTOR, A. TEACHING SUPERVISOR, ANDINSTRUCTORS. AN OUTLINE OF INFORMATIONAL MATERIAL TO BE PRESENTEDIN THE CLASSROOM, LABORATORY PROCEDURES TO BE DEMONSTRATEDAND THEN PERFORMED AS DIRECT EXERCISES BY THE STUDENTS, AS WELLAS RELEVANT BIBLIOGRAPHIES, AUDIOVISUAL AIDS, AND STUDYQUESTIONS ARE PRESENTED FOR THE FOLLOWING UNITS (1) ORIENTATION TO THE CLINICAL LABORATORY,(2) BACTERIOLOGY,(3) SEROLOGY, (4) PARASITOLOGY,(5) HEMATOLOGY, (6) CLINICAL CHEMISTRY,(7) BLOOD BANKING,(8) ROUTINE ANALYSIS, AND (9) BASAL METABOLISM -- ELECTROCARDIOGRAPHY. THIS DOCUMENT IS AVAILABLE AS GPO NUMBER FS 5.267-07017 FOR 60 CENTS FROMSUPERINTENDENT OF DOCUMENTS, U.S. GOVERNMENT PRINTING OFFICE,WASHINGTON, D.C. 20402. (PS) cir 02281"2=10. -

We Get Biased on Things That Make Sense
DIALOGUE AND DISCUSSION We Get Biased on Things That Make Sense MERIH T. TESFAZGHI When I was teaching third year medical students, and bench- reaction might be characterized by a marked left shift lecturing clinical laboratory science students, I emphasized which may display a range of immature cells that are also the importance of clinical details and the history of patients seen in CML. Severe left shift especially in the absence of in laboratory test processing, especially in the evaluation of evident infection (or other underlying diseases) may peripheral blood films. During those times, one of my strongly suggest direct bone marrow involvement. In students stated, “but including details of patients might such scenario, clinical details of patients such as absence somehow affect the decision of the reviewer, and may lead to or presence of enlargement of extramedullar organs is Downloaded from a bias.” I responded that we are biased on things that make highly sought. sense. This means that we decide based on the evidence we see from a microscopy evaluation in the light of the clinical Ø The coexistence of multiple conditions in a given detail and history. How do clinical details and history help us patient. One condition may mask the presence of the reach decisions? other condition. For example, the coexistence of megaloblastic anemia with iron deficiency anemia. http://hwmaint.clsjournal.ascls.org/ If specimens are the “in vitro ambassadors” of patients to the Usually, megaloblastic anemia is characterized by laboratory, then properly completed request forms are their increased size of red blood cells (MCV), but when “credentials”. -

20 Hemolytic Anemias Due to Abnormal Red Cell Enzymes
Hemolytic Anemias Due to Abnormal Red Cell Enzymes MODULE Hematology and Blood Bank Technique 20 HEMOLYTIC ANEMIAS DUE TO Notes ABNORMAL RED CELL ENZYMES 20.1 INTRODUCTION The main metabolic substrate for the RBCs is glucose. It is metabolized by two pathways: approximately 90% of the glucose is metabolized through the Embden Meyerhoff (glycolytic) pathway and the rest by the hexose monophosphate (HMP) pathway. In the Embden Meyerhoff (glycolytic) pathway glucose is metabolized to lactate through a series of enzymatic steps. Each molecule of glucose gives rise to 2 molecules of ATP. The ATP provides energy to maintain red cell volume, shape and flexibility. An ATP dependent pump in the red cell membrane actively keeps sodium out of the cell and potassium inside. The red cell has the enzymes that are needed for the glycolytic pathway. These enzymes help break down glucose to generate ATP which is the source of energy. About 10% of the glucose is diverted to the Hexose Monophosphate shunt pathway and this is essential for protection of red cells from oxidative stress. This pathway is necessary for the generation of NADPH which then reduces oxidized glutathione (GSSG) to reduced glutathione (GSH). GSH prevents the accumulation of H2O2 and the oxidation of hemoglobin to methemoglobin. When the level of GSH falls, H2O2 accumulates in the cell and oxidizes the hemoglobin to methemoglobin which becomes denatured and precipitates as Heinz bodies. These inclusions are rigid and attached to the red cell membrane and make the red cell susceptible to hemolysis. The NADPH required in this pathway is generated by the enzyme Glucose 6 phosphate dehydrogenase (G6PD). -
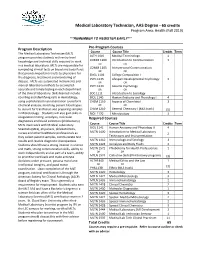
Medical Laboratory Technician 65 Credit AAS Program Guide
Medical Laboratory Technician, AAS Degree - 65 credits Program Area: Health (Fall 2019) ***REMEMBER TO REGISTER EARLY*** Program Description Pre-Program Courses Course Course Title Credits Term The Medical Laboratory Technician (MLT) program provides students with entry level ALTH 1410 Medical Terminology 1 knowledge and technical skills required to work COMM 1100 Introduction to Communication 3 in a medical laboratory. MLTs are responsible for OR OR COMM 1105 Interpersonal Communication completing clinical tests on blood and body fluids OR OR that provide important results to physicians for ENGL 1106 College Composition I the diagnosis, treatment and monitoring of PSYC 1135 Lifespan Developmental Psychology 3 disease. MLTs use automated instruments and OR OR manual laboratory methods to accomplish PSYC 1120 General Psychology accurate and timely testing in each department OR OR of the clinical laboratory. Skills learned include SOC 1111 Introduction to Sociology counting and identifying cells in Hematology, BIOL 1140 Human Anatomy and Physiology I 4 using sophisticated instrumentation to perform CHEM 1110 Aspects of Chemistry I 3 chemical analysis, matching patient blood types OR OR to donors for transfusion and preparing samples CHEM 1210 General Chemistry I (MLS track) (5) in Microbiology. Students will also gain skills in BIOL 1170 Microbiology 3 coagulation testing, urinalysis, molecular Required Courses diagnostics and blood collection (phlebotomy). MLTs must work with Medical Laboratory Course Course Title Credits Term Scientists (MLS), physicians, phlebotomists, BIOL 1141 Human Anatomy and Physiology II 4 MLTN 1400 Introduction to Medical Laboratory nurses and other healthcare professionals as 2 they collect patient samples, communicate test Techniques and Instrumentation results and resolve diagnostic discrepancies. -

Hematology Unit Lab 1 Review Material
Hematology Unit Lab 1 Review Material Objectives Laboratory instructors: 1. Facilitate lab discussion and answer questions Students: 1. Review the introductory material below 2. Study and review the assigned cases and questions in small groups before the Lab. This includes the pathological material using Virtual Microscopy 3. Be prepared to present your cases, questions and answers to the rest of your Lab class during the Lab Erythropoiesis: The process of red blood cell (RBC) production • Characterized by: − Increasing hemoglobin synthesis Erythroid maturation stages (Below): − Decreasing cell size - Average of 4 cell divisions during maturation − Decreasing cytoplasmic basophilia [One pronormoblast gives rise to 16 red cells] (increasing pink color) - pronormoblast → reticulocyte = 7 days − Progressive chromatin condensation of the - reticulocytes → mature RBC =1-2 days nuclei − Extrusion of nucleus (orthochromatic stage) − Extruded nuclei are subsequently phagocytized − Loss of mitotic capability after the early stage of polychromatophilic normoblast • Picture below: Erythroid progenitors (normoblasts) cluster around macrophages (arrows) in the bone marrow and spleen • Macrophages store iron • Iron is transferred from macrophages to erythroid precursor cells • Iron is used by normoblasts for hemoglobin synthesis aka nucleated rbc aka reticulocyte 1 Mature Red Blood Cell 7-8 microns; round / ovoid biconcave disc with orange-red cytoplasm, no RNA, no nucleus; survives ~120 days in circulation Classification of Anemia by Morphology 1. -

Laboratory Medicine: a National Status Report
Laboratory Medicine: A National Status Report Prepared for: Division of Laboratory Systems National Center for Preparedness, Detection, and Control of Infectious Diseases Centers for Disease Control and Prevention Prepared by: The Lewin Group Under subcontract to Battelle Memorial Institute May 2008 Laboratory Medicine: A National Status Report Prepared for: Division of Laboratory Systems National Center for Preparedness, Detection, and Control of Infectious Diseases Centers for Disease Control and Prevention Prepared by: The Lewin Group Julie Wolcott, MA Amanda Schwartz Clifford Goodman, PhD May 2008 Laboratory Medicine: A National Status Report Executive Summary TABLE OF CONTENTS ACKNOWLEDGMENTS v EXECUTIVE SUMMARY 1 The Value of Laboratory Medicine to Health Care ....................................................................... 2 Market Profile of the Laboratory Medicine Sector ........................................................................ 3 Laboratory Medicine Workforce...................................................................................................... 4 Quality and the Total Testing Process............................................................................................. 4 Quality Systems and Performance Measurement ......................................................................... 6 Laboratory Information Systems ..................................................................................................... 6 Federal Regulatory Oversight Of Laboratory Medicine.............................................................. -

Copyrighted Material
CHAPTER 1 The Blood Film and Count Blood Blood is a life‐sustaining fluid that circulates through the heart and blood vessels. It carries oxygen and nutrients to the tissues and waste products to the lungs, liver and kidneys, where they can be removed from the body. Usually when blood is removed from the body it forms a solid blood clot. However, if clotting is prevented by mixing with an anticoagulant, the blood separates, under the influence of gravity, into three layers (Fig. 1.1). The bottom layer is deep red in colour and is composed of red cells. The top layer is clear and pale yellow. It is called plasma and is composed of various salts and proteins dissolved in water. In between is a narrow layer called the buffy coat because of its buff or yellowish white colour. The buffy coat is composed mainly of cells of a variety of types, collectively known as white cells. In addition there are small cellular fragments, called platelets, which have a role in blood clotting. The bloodCOPYRIGHTED film MATERIAL Although we can judge the proportions of red cells and white cells in a tube of sedimented blood, we get far more information if the blood is carefully mixed and a thin layer is spread on a glass A Beginner’s Guide to Blood Cells, Third Edition. Barbara J. Bain. © 2017 John Wiley & Sons Ltd. Published 2017 by John Wiley & Sons Ltd. 1 0003049575.indd 1 2/24/2017 9:50:31 AM 2 A Beginner’s Guide to Blood Cells Plasma Buffy coat Red cells Fig. -

SEED Haematology Sysmex Educational Enhancement and Development October 2012
SEED Haematology Sysmex Educational Enhancement and Development October 2012 The red blood cell indices The full blood count has been used in conjunction with the traditional red The complete blood count (CBC) is central to clinical deci- cell indices in order to narrow down the possible causes sion making. This makes it one of the commonest laboratory of anaemia in an individual patient. investigations performed worldwide. Whilst the definition of what constitutes an CBC is influenced by the number Impedance technology and type of parameters measured by different haematology The RBC, HCT and MCV are all closely interrelated as they analysers, the traditional red cell indices that are widely are derived from information obtained from the passage used to classify anaemias are common to all. of cells through the aperture of the impedance channel of an automated haematology analyser. The impedance The laboratory approach to anaemia technology is based on the principle that an electrical field, Anaemia is an extremely common global healthcare prob- created between two electrodes of opposite charge, can lem. However, anaemia is merely a symptom which can be used to count and determine the size of cells. Blood result from a multitude of causes. Effective treatment is cells are poor conductors of electricity. The diluent in which only possible if the underlying cause is correctly identified. they are suspended as they pass through the aperture To this end, several classification systems have been devis- during counting is an isotonic solution which is a good ed. The most useful and widely used classification system conductor of electricity. -

Blood Film Preparation and Staining Procedures
INTERPRETATION OF THE PERIPHERAL 00. ם BLOOD FILM 0272–2712/02 $15.00 BLOOD FILM PREPARATION AND STAINING PROCEDURES Berend Houwen, MD, PhD The blood film is one of the world’s most widely and frequently used tests and has undergone remarkably few changes since its introduction as a clinical diagnostic tool in the late 1800s. The origins of stained blood film microscopy are somewhat obscure, and a clear reference for the ‘‘first person or persons’’ to describe its use as a clinical laboratory procedure cannot be established with certainty. Antonie van Leeuwenhoek, in the seventeenth century, was the first to describe blood cells, using whole blood preparations and not blood films for his observations on blood corpuscles. In fact, he would not have been able to use blood film–based microscopy because it requires considerably more sophisti- cated optics than were available in his day. A second technologic innovation enabling blood film microscopy was the introduction of aniline dyes in the second half of the nineteenth century. This made it possible to study individual blood cells by light microscopy after a small amount of blood had been placed and smeared onto glass slides, dried, fixed, and then stained. Paul Ehrlich introduced eosin as the first of these dyes for staining blood films in 1856, followed by hematoxylin in 1865, and later by the metachromatic Romanowsky dyes. A few refinements have since been made, consisting mainly of improve- ments in dye quality, staining procedures, automation of slide preparation, and staining, but the basic elements of blood film preparation and analysis have not changed for over a century. -

The Widespread Application of Red Cell Survival Sive Red
CLINICAL DETERMINATION OF THE SITES OF RED CELL SEQUESTRATION IN HEMOLYTIC ANEMIAS1 By JAMES H. JANDL, MORTIMER S. GREENBERG, ROBERT H. YONEMOTO, AND WILLIAM B. CASTLE (From the Thorndike Memorial Laboratory and Second and Fourth (Harvard), Medical Services Boston City Hospital, and the Department of Medicine, Harvard Medical School, Boston, Mass.) (Submitted for publication January 30, 1956; accepted April 3, 1956) The widespread application of red cell survival greater than that of other tissues even when cor- techniques has revealed the importance of exces- rection was made for the Cr5l activity of the re- sive red cell destruction in the pathologic physi- sidual red cells. Moreover, the radioactivity of ology of many of the anemias. An increasing ar- the packed red cells removed from the spleen ex- ray of in vitro methods for detecting red cell or ceeded that of a comparable sample of packed red serum abnormalities has provided insight into the cells from the peripheral blood. In order to in- in vivo mechanisms underlying some of these proc- vestigate the possibility that Cr51-labelled red cell esses. In certain disease states the presence of deposition could be determined by measuring body visible or physically measurable alterations of the surface radioactivity, several questions required red cells has permitted detection of the sites and exploration: 1) Are the emanations of Cr5 suit- to some extent of the mechanisms of sequestration able for external body scanning at safe dosage of these cells. Such valuable observations have levels? 2) Does the site of tissue deposition of been made upon pathologic material from patients Cr65 following the intravenous injection of Cr51- with congenital hemolytic anemia (1-5) and labelled red cells necessarily indicate the site of sickle cell anemia (1, 5-7).