Prevalence and Instability of Fragile X Alleles Implications for Offering Fragile X Prenatal Diagnosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Case-Scenario-10-FINAL.Pdf
Case scenario 10 – Magdalena Part 1 • Magdalena is 26 years old. She is 13 weeks pregnant and had a high chance combined test result of 1 in 140 for trisomy 13 with an NT measurement of 2.4mm. • After a lengthy discussion about her options, she decided to have NIPT. • The results reported a greater than 60% chance of the baby having Patau’s syndrome. She decided to have an invasive test. A CVS was performed. Would you have offered a CVS to this patient? a) No - she should be offered an amniocentesis only, due to the chance of placental factors affecting a result. b) Yes - the patient should be informed of risks and benefits of both CVS and amniocentesis. Answer b) Yes - the patient should be informed of the risks and benefits of a CVS and amniocentesis. Additional note A patient/couple should be informed of the options of both forms of diagnostic procedures. They should be informed of the benefits and limitations of each test, this should include timing of testing, possible results and risks of miscarriage. This discussion should also include the couples ethical, religious, social and individual beliefs. Name the type of mosaicism that can cause a false-positive result with NIPT. a) Feto-placental mosaicism b) Placental mosaicism c) Fetal mosaicism Answer b) Placental mosaicism Additional note There is a discrepancy of the cell line in the placenta and baby. The abnormal cell lines are seen in the placenta and not on the fetus. There is a small chance that a 'high chance report is caused by 'placental mosaicism', therefore a CVS may also report 'mosaicism’. -

Survivors of Acute Leukemia Are Less Likely to Have Liveborn Infants Than Are Their
CHILDHOOD CANCER SURVIVOR STUDY ANALYSIS PROPOSAL STUDY TITLE: Fertility Rates in Long-Term Survivors of Acute Lymphoblastic Leukemia WORKING GROUP AND INVESTIGATORS: Name Telephone Number E-mail Daniel M. Green, M.D. 901-595-5915 [email protected] Vikki Nolan, Ph.D. 901-595-6078 [email protected] Liang Zhu, Ph.D. 901-595-5240 [email protected] Marilyn Stovall, Ph.D. 713-792-3240 [email protected] Sarah Donaldson, M.D. 650-723-6195 [email protected] Les Robison, Ph.D. 901-595-5817 [email protected] Chuck Sklar, M.D. 212-717-3239 [email protected] BACKGROUND AND RATIONALE: Survivors of acute leukemia are less likely to have liveborn infants than are their female siblings (relative risk (RR) =0.63, 95% confidence interval (CI) 0.52 to 0.76). The risk of miscarriage was increased among Childhood Cancer Survivor Study (CCSS) female participants who received craniospinal (RR=2.22, 95% CI 1.36 to 3.64) or cranial irradiation (RR=1.40, 95% CI 1.02 to 1.94). The risk of miscarriage was increased in survivors of acute lymphoblastic leukemia (ALL) (RR=1.60, 95% CI 0.85 to 3.00) and central nervous system tumors (RR=1.33, 95% CI 0.61 to 2.93) although neither risk achieved statistical significance 1. Winther et al. reported that the risk of spontaneous 2 abortion was not increased in survivors of leukemia compared to their sisters (proportion ratio (PR) 1.2, 95% CI 0.7 to 2.0). However those female survivors who received low doses of radiation to the uterus and ovaries, but high doses of radiation to the pituitary had an increased risk of spontaneous abortion (PR 1.8, 95% CI 1.1 to 3.0). -

Role of Maternal Age and Pregnancy History in Risk of Miscarriage
RESEARCH Role of maternal age and pregnancy history in risk of BMJ: first published as 10.1136/bmj.l869 on 20 March 2019. Downloaded from miscarriage: prospective register based study Maria C Magnus,1,2,3 Allen J Wilcox,1,4 Nils-Halvdan Morken,1,5,6 Clarice R Weinberg,7 Siri E Håberg1 1Centre for Fertility and Health, ABSTRACT Miscarriage and other pregnancy complications might Norwegian Institute of Public OBJECTIVES share underlying causes, which could be biological Health, PO Box 222 Skøyen, To estimate the burden of miscarriage in the conditions or unmeasured common risk factors. N-0213 Oslo, Norway Norwegian population and to evaluate the 2MRC Integrative Epidemiology associations with maternal age and pregnancy history. Unit at the University of Bristol, Introduction Bristol, UK DESIGN 3 Miscarriage is a common outcome of pregnancy, Department of Population Prospective register based study. Health Sciences, Bristol Medical with most studies reporting 12% to 15% loss among School, Bristol, UK SETTING recognised pregnancies by 20 weeks of gestation.1-4 4Epidemiology Branch, National Medical Birth Register of Norway, the Norwegian Quantifying the full burden of miscarriage is Institute of Environmental Patient Register, and the induced abortion register. challenging because rates of pregnancy loss are Health Sciences, Durham, NC, USA PARTICIPANTS high around the time that pregnancies are clinically 5Department of Clinical Science, All Norwegian women that were pregnant between recognised. As a result, the total rate of recognised University of Bergen, Bergen, 2009-13. loss is sensitive to how early women recognise their Norway pregnancies. There are also differences across countries 6 MAIN OUTCOME MEASURE Department of Obstetrics and studies in distinguishing between miscarriage and and Gynecology, Haukeland Risk of miscarriage according to the woman’s age and University Hospital, Bergen, pregnancy history estimated by logistic regression. -

Practice Guidelines for Molecular Diagnosis of Fragile X Syndrome
Practice Guidelines for Molecular Diagnosis of Fragile X Syndrome Prepared and edited by James Macpherson 1 and Abid Sharif 2 following a CMGS Workshop held on 10 th July 2012. 1. Wessex Regional Genetics Laboratory, Salisbury NHS Foundation Trust, Salisbury, Wiltshire, SP2 8BJ, U.K. 2. East Midlands Regional Molecular Genetics Service, Nottingham University Hospitals NHS Trust, City Hospital Campus, Nottingham, NG5 1PB, U.K. Guidelines updated by the Association for Clinical Genetic Science (formally Clinical Molecular Genetics Society and Association of Clinical Cytogenetics) approved November 2014. 1. NOMENCLATURE and GENE IDs OMIM Condition Gene name Gene map locus 309550 Fragile X Syndrome FMR1 Xq27.3 309548 FRAXE FMR2 Xq28 2. DESCRIPTION OF DISEASE 2.1 Fragile X Syndrome Fragile X Syndrome is thought to be the commonest single-gene cause of learning disability features in humans with an estimated prevalence of 1 in 4000- 1 in 6000 males, where it causes moderate to severe intellectual and social impairment together with syndromic features including large ears and head, long face and macroorchidism 1. A fragile site (FRAXA) is expressible at the gene locus at Xq27.3, typically in 2-40 % of blood cells in affected males. The pathogenic mutation in most cases is a large expansion (‘full mutation’) in a CGG repeat tract in the first untranslated exon of the gene FMR1, which normally encodes the RNA-binding protein FMRP. Full mutations (from approximately 200 repeats upwards) result in hypermethylation of the DNA in and around the CGG tract, curtailed gene expression and no FMRP being produced 2-4. Smaller expansions of the CGG repeat, or ‘premutations’ are not hypermethylated and hence do not cause Fragile X syndrome, but may show expansion into full mutations over one or more generations. -

ART and Miscarriage Risk Assoc
27-28 January, Sofia, Bulgaria ART and miscarriage risk Assoc. Prof. Petya Andreeva, MD, PhD D-r Shterev Hospital Dr. Shterev Sofia, BULGARIA HOSPITAL Dr. Shterev Miscarriage rate HOSPITAL 10 % to 15 % of clinical pregnancies have resulted in miscarriage. 1-2% miscarriages / per couples who try to conceive. (Macklon NS et al, 2002; Rai R et al 2006) Dr. Shterev Monthly fecundity rate (MFR) HOSPITAL In humans even in optimal circumstances – clinical recognized pregnancy in one cycle or the so called monthly fecundity rate is around 30 % . In contrast MFR is 80% in baboons and 90% in rabbits. (Chard T, 1991; . Foote RH 1988; Stevens VC 1997) Dr. Shterev Ongoing pregnancy rate HOSPITAL Assisted reproductive technologies (ART) represent average 30 % pregnancy rate. Around 50% of human conception fails implantation. Up to half of implanted embryos fail to progress in ongoing pregnancy. (Macklon N 2002; Macklon N 2014) Dr. Shterev Conception to ongoing pregnancy HOSPITAL Live births -True incidence of pregnancy loss is 30% closer to 50%. Miscarriage - This renders miscarriage as the 40-50% 10 % most common complication of pregnancy Early pregnancy loss 30 % Implantation failure 30 % CONCEPTION Macklon et al, Hum Reproduction Update, 2002 Dr. Shterev Known reasons for miscarriage HOSPITAL Antiphospholipid syndrome Endocrine abnormalities Thyroid dysfunction Diabetes Chromosome aberrations Uterine structural malformation Trombophilias Unknown factors in 50 % of cases. Dr. Shterev Embryo HOSPITAL The enormous rate of early pregnancy loss in humans thought to be as a consequences of two key features of human embryos: 1. High prevalence of chromosomal abnormalities. 2. Invasiveness. Dr. Shterev HOSPITAL -Good-quality cleavage-stage embryos exhibit high rates of aneuploidy. -

Biases Inherent in Studies of Coffee Consumption in Early Pregnancy and the Risks of Subsequent Events
nutrients Review Biases Inherent in Studies of Coffee Consumption in Early Pregnancy and the Risks of Subsequent Events Alan Leviton ID Boston Children’s Hospital and Harvard Medical School, 1731 Beacon Street, Brookline, MA 02445, USA; [email protected]; Tel.: +1-617-485-7187 Received: 24 July 2018; Accepted: 21 August 2018; Published: 23 August 2018 Abstract: Consumption of coffee by women early in their pregnancy has been viewed as potentially increasing the risk of miscarriage, low birth weight, and childhood leukemias. Many of these reports of epidemiologic studies have not acknowledged the potential biases inherent in studying the relationship between early-pregnancy-coffee consumption and subsequent events. I discuss five of these biases, recall bias, misclassification, residual confounding, reverse causation, and publication bias. Each might account for claims that attribute adversities to early-pregnancy-coffee consumption. To what extent these biases can be avoided remains to be determined. As a minimum, these biases need to be acknowledged wherever they might account for what is reported. Keywords: epidemiology; bias; causation; coffee; pregnancy 1. Introduction Maternal consumption of coffee during early pregnancy has been viewed as increasing the risk of miscarriage [1–4], fetal growth restriction [2,5–11], and childhood leukemias [12–20]. Unfortunately, many of the epidemiologic studies have not acknowledged the potential biases that appear to have influenced these perceptions of risk. The list of potential biases is long [21]. In this essay, I review five of these biases, namely recall bias, misclassification, residual confounding, reverse causation, and publication bias. Each of these biases might account for some of what has been reported. -

FDA Requests Removal of Strongest Warning Against Using Cholesterol
FDA requests removal of strongest warning against using cholesterol-lowering statins during pregnancy; still advises most pregnant patients should stop taking statins Breastfeeding not recommended in patients who require statins 7-20-2021 FDA Drug Safety Communication What safety information is FDA announcing? The U.S. Food and Drug Administration (FDA) is requesting removal of its strongest warning against using cholesterol-lowering statin medicines in pregnant patients. Despite the change, most patients should stop statins once they learn they are pregnant. We have conducted a comprehensive review of all available data and are requesting that statin manufacturers make this change to the prescribing information as part of FDA’s ongoing effort to update the pregnancy and breastfeeding information for all prescription medicines. Patients should not breastfeed when taking a statin because the medicine may pass into breast milk and pose a risk to the baby. Many can stop statins temporarily until breastfeeding ends. However, patients requiring ongoing statin treatment should not breastfeed and instead use infant formula or other alternatives. What is FDA doing? We are requesting revisions to the information about use in pregnancy in the prescribing information of the entire class of statin medicines. These changes include removing the contraindication against using these medicines in all pregnant patients. A contraindication is FDA’s strongest warning and is only added when a medicine should not be used because the risk clearly outweighs any possible benefit. Because the benefits of statins may include prevention of serious or potentially fatal events in a small group of very high-risk pregnant patients, contraindicating these drugs in all pregnant women is not appropriate. -

Maternal Age, History of Miscarriage, and Embryonic/Fetal Size Are Associated with Cytogenetic Results of Spontaneous Early Miscarriages
Journal of Assisted Reproduction and Genetics (2019) 36:749–757 https://doi.org/10.1007/s10815-019-01415-y GENETICS Maternal age, history of miscarriage, and embryonic/fetal size are associated with cytogenetic results of spontaneous early miscarriages Nobuaki Ozawa1 & Kohei Ogawa1 & Aiko Sasaki1 & Mari Mitsui1 & Seiji Wada1 & Haruhiko Sago1 Received: 1 October 2018 /Accepted: 28 January 2019 /Published online: 9 February 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Purpose To clarify the associations of the maternal age, history of miscarriage, and embryonic/fetal size at miscarriage with the frequencies and profiles of cytogenetic abnormalities detected in spontaneous early miscarriages. Methods Miscarriages before 12 weeks of gestation, whose karyotypes were evaluated by G-banding between May 1, 2005, and May 31, 2017, were included in this study. The relationships between their karyotypes and clinical findings were assessed using trend or chi-square/Fisher’s exact tests and multivariate logistic analyses. Results Three hundred of 364 miscarriage specimens (82.4%) had abnormal karyotypes. An older maternal age was significantly associated with the frequency of abnormal karyotype (ptrend < 0.001), particularly autosomal non-viable and viable trisomies (ptrend 0.001 and 0.025, respectively). Women with ≥ 2 previous miscarriages had a significantly lower possibility of miscarriages with abnormal karyotype than women with < 2 previous miscarriages (adjusted odds ratio [aOR], 0.48; 95% confidence interval [95% CI], 0.27–0.85). Although viable trisomy was observed more frequently in proportion to the increase in embryonic/fetal size at miscarriage (ptrend < 0.001), non-viable trisomy was observed more frequently in miscarriages with an embryonic/fetal size < 10 mm (aOR, 2.41; 95% CI, 1.27–4.58), but less frequently in miscarriages with an embryonic/fetal size ≥ 20 mm (aOR, 0.01; 95% CI, 0.00–0.07) than in anembryonic miscarriages. -

Miscarriage in Early Pregnancy
Miscarriage in Early Pregnancy Obstetrics & Gynaecology Women and Children’s Group This leaflet has been designed to give you important information about your condition / procedure, and to answer some common queries that you may have. Introduction What has happened? This booklet has been written to give help Bleeding from the vagina in early pregnancy and guidance to parents who lose a baby in is very common. Most pregnancies will the early stages of pregnancy. continue as normal but sadly other Parents who have suffered such a loss by pregnancies will end in miscarriage. miscarriage find that they need to make a Miscarriage is the term used to describe the number of choices within a short space of sudden ending of a pregnancy, most often time, choices that they may rather not think within the first 12 weeks. about. With the help of the staff and the information in this booklet, we can help you Inevitable miscarriage through your period of grief, making this Some women find that the initial bleeding stressful time easier to cope with. becomes heavier, sometimes with blood At the moment you may be experiencing clots. There may also be severe period-type feelings of anxiety, distress and sadness. pains or cramps. What is happening is that Grief is a very natural reaction to the loss of the uterus is trying to push out, or expel, the your baby, and grief following a miscarriage pregnancy. may be just as strong as that occurring after the loss of someone we have known and Incomplete miscarriage loved. How a particular person copes with This is when the pregnancy is partially grief is unique to that person. -

Genetics of Recurrent and Spontaneous Miscarriage
Research and Reviews Journal of Medical and Health Science ISSN: 2319-9865 Genetics of Recurrent and Spontaneous Miscarriage Arka Dutta Gupta1* and Akash Baid2 1 Department of Human Genetics, Institute of Genetic Engineering, Madhyamgram, Badu, Itkhola, Kolkata, West Bengal 2 Department of Biotechnology, Institute of Genetic Engineering, Madhyamgram, Badu, Itkhola, Kolkata, West Bengal Research Article Received: 01/03/2017 ABSTRACT Revised: 20/03/2017 Accepted: 27/03/2017 The aim was to find out the genetic basis of recurrent spontaneous abortion (RSA) from the past pregnancies and ensure a more favourable *For Correspondence outcome in the current or future pregnancy. Pregnancy loss has always been Gupta AD, Department of Human a devastating experience for the mothers and the clinician of concern. One Genetics, Institute of Genetic out of four pregnancies ends in miscarriage. It is estimated that 50-60% of Engineering, Madhyamgram, all first trimester pregnancy losses are the cause of chromosomal Badu, Itkhola, Kolkata, West abnormality. Bengal, Tel: 8978764411. 8 couples were selected with the history of Recurrent Spontaneous fetal loss and were suggested ultrasound scan while no such abnormalities Keywords: Recurrent were found, hence further we asked for cytogenetic procedure for detection spontaneous miscarriage, Bad of chromosomal abnormalities by the method karyotyping. Obstetric History, chromosomal We discovered 45;X/46;XX, 45;X/46;XY and 46;XX/46;XY in female abnormalities partners and 46;XX/46;XY, 46;XY/47;XXY mosaicism in male partners. Two unique balanced translocations in females such as 46;XX,t(5q35:8q24) E-mail: and46;XX,t(14q:21q) and a single balanced translocation in male partner [email protected] 46;XY,t(6q:8p) with reproductive failure. -

HTSP 101: Everything You Want to Know About Healthy Timing and Spacing of Pregnancy
HTSP 101: Everything You Want to Know About Healthy Timing and Spacing of Pregnancy Healthy Timing and Spacing of Pregnancy (HTSP) is an intervention to help women and families delay or space their pregnancies to achieve the healthiest outcomes for women, newborns, infants, and children, within the context of free and informed choice, taking into account fertility intentions and desired family size. Background Over the past few years, the United States Agency Qualitative studies conducted by USAID in for International Development (USAID) has Pakistan, India, Bolivia, and Peru showed that sponsored a series of studies on pregnancy spacing women and couples are interested in the healthiest and health outcomes. The research objective was to time to become pregnant versus when to give birth. assess, from the best available evidence, the effects In this way, HTSP differs from previous birth of pregnancy spacing on maternal, newborn and spacing approaches that refer only to the interval child health outcomes. In June 2005, the World after a live birth and when to give birth. HTSP also Health Organization (WHO) convened a panel of 30 provides guidance on the healthiest age for the first technical experts to review six USAID-sponsored pregnancy. studies. Based on their review of the evidence, the technical experts made two recommendations* to the Thus, HTSP encompasses a broader concept of the WHO, which are included in a report and policy reproductive cycle starting from healthiest age for brief1: the first pregnancy in adolescents, to spacing subsequent pregnancies following a live birth, still • After a live birth, the recommended minimum birth, miscarriage or abortion – capturing all interval before attempting the next pregnancy is pregnancy-related intervals in a woman’s at least 24 months in order to reduce the risk of reproductive life. -
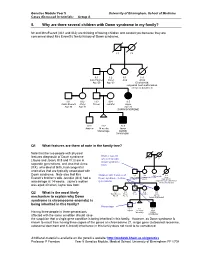
5. Why Are There Several Children with Down Syndrome in My Family?
Genetics Module Year 5 University of Birmingham, School of Medicine Cases discussed in tutorials: Group A 5. Why are there several children with Down syndrome in my family? Mr and Mrs Everett (III:1 and III:2) are thinking of having children and consult you because they are concerned about Mrs Everett’s family history of Down syndrome. I:1 I:2 II:1 II:2 II:3 II:4 Alan Hughes Carol Julia Anna Age 60 Age 60 Died at birth: congenital heart malformation, atresia of duodenum III:1 III:2 III:3 III:4 III:5 Gavin Everett Meryl Trevor Jennifer Jayne Age 35 Age 32 Age 20 DOWN SYNDROME IV:1 IV:2 IV:3 Andrew 14 weeks Jason Miscarriage DOWN SYNDROME Q1 What features are there of note in the family tree? Note that the two people with physical features diagnostic of Down syndrome Mother was 40 (Jayne and Jason; III:5 and IV:3) are in when child with I:1 I:2 Down syndrome separate generations, and also that Anna born (II:4), who died at birth, had congenital anomalies that are typically associated with Down syndrome. Note also that Mrs Children with features of II:1 II:2 II:3 II:4 Alan Hughes Carol Julia Anna Everett’s brother’s wife Jennifer (III:4) had a Down syndrome in three Age 60 Age 60 Died at birth: generations congenital heart malformation miscarriage at 14 weeks. Jayne’s mother atresia of duodenum was aged 40 when Jayne was born. III:1 III:2 III:3 III:4 III:5 Q2 What is the most likely Gavin Everett Meryl Trevor Jennifer Jayne Age 35 Age 32 Age 20 mechanism to explain why Down DOWN SYNDROME syndrome (a chromosome anomaly) is being inherited in this family? Miscarriage IV:1 IV:2 IV:3 Andrew 14 weeks Jason Having three people in three generations Miscarriage DOWN affected with the same condition should raise SYNDROME the suspicion that a single gene condition is being inherited in this family.