A Simple Way to Test for Collinearity in Spin Symmetry Broken Wave Functions: General Theory and Application to Generalized Hartree Fock
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Projective Geometry: a Short Introduction
Projective Geometry: A Short Introduction Lecture Notes Edmond Boyer Master MOSIG Introduction to Projective Geometry Contents 1 Introduction 2 1.1 Objective . .2 1.2 Historical Background . .3 1.3 Bibliography . .4 2 Projective Spaces 5 2.1 Definitions . .5 2.2 Properties . .8 2.3 The hyperplane at infinity . 12 3 The projective line 13 3.1 Introduction . 13 3.2 Projective transformation of P1 ................... 14 3.3 The cross-ratio . 14 4 The projective plane 17 4.1 Points and lines . 17 4.2 Line at infinity . 18 4.3 Homographies . 19 4.4 Conics . 20 4.5 Affine transformations . 22 4.6 Euclidean transformations . 22 4.7 Particular transformations . 24 4.8 Transformation hierarchy . 25 Grenoble Universities 1 Master MOSIG Introduction to Projective Geometry Chapter 1 Introduction 1.1 Objective The objective of this course is to give basic notions and intuitions on projective geometry. The interest of projective geometry arises in several visual comput- ing domains, in particular computer vision modelling and computer graphics. It provides a mathematical formalism to describe the geometry of cameras and the associated transformations, hence enabling the design of computational ap- proaches that manipulates 2D projections of 3D objects. In that respect, a fundamental aspect is the fact that objects at infinity can be represented and manipulated with projective geometry and this in contrast to the Euclidean geometry. This allows perspective deformations to be represented as projective transformations. Figure 1.1: Example of perspective deformation or 2D projective transforma- tion. Another argument is that Euclidean geometry is sometimes difficult to use in algorithms, with particular cases arising from non-generic situations (e.g. -

Robot Vision: Projective Geometry
Robot Vision: Projective Geometry Ass.Prof. Friedrich Fraundorfer SS 2018 1 Learning goals . Understand homogeneous coordinates . Understand points, line, plane parameters and interpret them geometrically . Understand point, line, plane interactions geometrically . Analytical calculations with lines, points and planes . Understand the difference between Euclidean and projective space . Understand the properties of parallel lines and planes in projective space . Understand the concept of the line and plane at infinity 2 Outline . 1D projective geometry . 2D projective geometry ▫ Homogeneous coordinates ▫ Points, Lines ▫ Duality . 3D projective geometry ▫ Points, Lines, Planes ▫ Duality ▫ Plane at infinity 3 Literature . Multiple View Geometry in Computer Vision. Richard Hartley and Andrew Zisserman. Cambridge University Press, March 2004. Mundy, J.L. and Zisserman, A., Geometric Invariance in Computer Vision, Appendix: Projective Geometry for Machine Vision, MIT Press, Cambridge, MA, 1992 . Available online: www.cs.cmu.edu/~ph/869/papers/zisser-mundy.pdf 4 Motivation – Image formation [Source: Charles Gunn] 5 Motivation – Parallel lines [Source: Flickr] 6 Motivation – Epipolar constraint X world point epipolar plane x x’ x‘TEx=0 C T C’ R 7 Euclidean geometry vs. projective geometry Definitions: . Geometry is the teaching of points, lines, planes and their relationships and properties (angles) . Geometries are defined based on invariances (what is changing if you transform a configuration of points, lines etc.) . Geometric transformations -
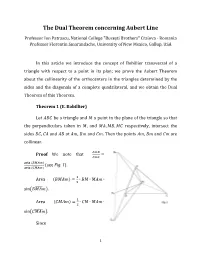
The Dual Theorem Concerning Aubert Line
The Dual Theorem concerning Aubert Line Professor Ion Patrascu, National College "Buzeşti Brothers" Craiova - Romania Professor Florentin Smarandache, University of New Mexico, Gallup, USA In this article we introduce the concept of Bobillier transversal of a triangle with respect to a point in its plan; we prove the Aubert Theorem about the collinearity of the orthocenters in the triangles determined by the sides and the diagonals of a complete quadrilateral, and we obtain the Dual Theorem of this Theorem. Theorem 1 (E. Bobillier) Let 퐴퐵퐶 be a triangle and 푀 a point in the plane of the triangle so that the perpendiculars taken in 푀, and 푀퐴, 푀퐵, 푀퐶 respectively, intersect the sides 퐵퐶, 퐶퐴 and 퐴퐵 at 퐴푚, 퐵푚 and 퐶푚. Then the points 퐴푚, 퐵푚 and 퐶푚 are collinear. 퐴푚퐵 Proof We note that = 퐴푚퐶 aria (퐵푀퐴푚) (see Fig. 1). aria (퐶푀퐴푚) 1 Area (퐵푀퐴푚) = ∙ 퐵푀 ∙ 푀퐴푚 ∙ 2 sin(퐵푀퐴푚̂ ). 1 Area (퐶푀퐴푚) = ∙ 퐶푀 ∙ 푀퐴푚 ∙ 2 sin(퐶푀퐴푚̂ ). Since 1 3휋 푚(퐶푀퐴푚̂ ) = − 푚(퐴푀퐶̂ ), 2 it explains that sin(퐶푀퐴푚̂ ) = − cos(퐴푀퐶̂ ); 휋 sin(퐵푀퐴푚̂ ) = sin (퐴푀퐵̂ − ) = − cos(퐴푀퐵̂ ). 2 Therefore: 퐴푚퐵 푀퐵 ∙ cos(퐴푀퐵̂ ) = (1). 퐴푚퐶 푀퐶 ∙ cos(퐴푀퐶̂ ) In the same way, we find that: 퐵푚퐶 푀퐶 cos(퐵푀퐶̂ ) = ∙ (2); 퐵푚퐴 푀퐴 cos(퐴푀퐵̂ ) 퐶푚퐴 푀퐴 cos(퐴푀퐶̂ ) = ∙ (3). 퐶푚퐵 푀퐵 cos(퐵푀퐶̂ ) The relations (1), (2), (3), and the reciprocal Theorem of Menelaus lead to the collinearity of points 퐴푚, 퐵푚, 퐶푚. Note Bobillier's Theorem can be obtained – by converting the duality with respect to a circle – from the theorem relative to the concurrency of the heights of a triangle. -

The Generalization of Miquels Theorem
THE GENERALIZATION OF MIQUEL’S THEOREM ANDERSON R. VARGAS Abstract. This papper aims to present and demonstrate Clifford’s version for a generalization of Miquel’s theorem with the use of Euclidean geometry arguments only. 1. Introduction At the end of his article, Clifford [1] gives some developments that generalize the three circles version of Miquel’s theorem and he does give a synthetic proof to this generalization using arguments of projective geometry. The series of propositions given by Clifford are in the following theorem: Theorem 1.1. (i) Given three straight lines, a circle may be drawn through their intersections. (ii) Given four straight lines, the four circles so determined meet in a point. (iii) Given five straight lines, the five points so found lie on a circle. (iv) Given six straight lines, the six circles so determined meet in a point. That can keep going on indefinitely, that is, if n ≥ 2, 2n straight lines determine 2n circles all meeting in a point, and for 2n +1 straight lines the 2n +1 points so found lie on the same circle. Remark 1.2. Note that in the set of given straight lines, there is neither a pair of parallel straight lines nor a subset with three straight lines that intersect in one point. That is being considered all along the work, without further ado. arXiv:1812.04175v1 [math.HO] 11 Dec 2018 In order to prove this generalization, we are going to use some theorems proposed by Miquel [3] and some basic lemmas about a bunch of circles and their intersections, and we will follow the idea proposed by Lebesgue[2] in a proof by induction. -

Linear Features in Photogrammetry
Linear Features in Photogrammetry by Ayman Habib Andinet Asmamaw Devin Kelley Manja May Report No. 450 Geodetic Science and Surveying Department of Civil and Environmental Engineering and Geodetic Science The Ohio State University Columbus, Ohio 43210-1275 January 2000 Linear Features in Photogrammetry By: Ayman Habib Andinet Asmamaw Devin Kelley Manja May Report No. 450 Geodetic Science and Surveying Department of Civil and Environmental Engineering and Geodetic Science The Ohio State University Columbus, Ohio 43210-1275 January, 2000 ABSTRACT This research addresses the task of including points as well as linear features in photogrammetric applications. Straight lines in object space can be utilized to perform aerial triangulation. Irregular linear features (natural lines) in object space can be utilized to perform single photo resection and automatic relative orientation. When working with primitives, it is important to develop appropriate representations in image and object space. These representations must accommodate for the perspective projection relating the two spaces. There are various options for representing linear features in the above applications. These options have been explored, and an optimal representation has been chosen. An aerial triangulation technique that utilizes points and straight lines for frame and linear array scanners has been implemented. For this task, the MSAT (Multi Sensor Aerial Triangulation) software, developed at the Ohio State University, has been extended to handle straight lines. The MSAT software accommodates for frame and linear array scanners. In this research, natural lines were utilized to perform single photo resection and automatic relative orientation. In single photo resection, the problem is approached with no knowledge of the correspondence of natural lines between image space and object space. -

1.3 Collinear, Betweenness, and Assumptions Date: Goals: How Are Diagrams Interpreted in the Study of Geometry
1.3 Collinear, Betweenness, and Assumptions Date: Goals: How are diagrams interpreted in the study of geometry Collinear/Noncollinear: Three or more points that lie on the same line are collinear. Points that do not lie on the same line are noncollinear. Two points are always collinear. For a point to be between two other points, all three points must be collinear. Example 1: Use the diagram to the right to answer the following questions. a. Are points A, B, and C collinear? A Yes, B is between A and C. b. Is point B between A and D? B No, the three points are noncollinear. c. Name 3 noncollinear points. AEC, , and E D C d. Are points A and D collinear? Yes, two points are always collinear. The Triangle Inequality: For any three points, there are only two possibilies: 1. They are collinear. One point must be between the other two. Two of the distances must add to the third. 2. They are noncollinear. The three points will form a triangle. Any two of the lengths will add to be larger than the third. Example 2: If AB=12, BC=13, and AC=25, can we determine if the points are collinear? Yes, the lengths 12 and 13 add to 25, therefore the points are collinear. How to Interpret a Diagram: You Should Assume: You Should Not Assume: Straight lines and angles Right Angles Collinearity of points Congruent Segments Betweenness of points Congruent Angles Relative position of points Relative sizes of segments and angles Example 3: Use the diagram to the right to answer the following questions. -

Dissertation Hyperovals, Laguerre Planes And
DISSERTATION HYPEROVALS, LAGUERRE PLANES AND HEMISYSTEMS { AN APPROACH VIA SYMMETRY Submitted by Luke Bayens Department of Mathematics In partial fulfillment of the requirements For the Degree of Doctor of Philosophy Colorado State University Fort Collins, Colorado Spring 2013 Doctoral Committee: Advisor: Tim Penttila Jeff Achter Willem Bohm Chris Peterson ABSTRACT HYPEROVALS, LAGUERRE PLANES AND HEMISYSTEMS { AN APPROACH VIA SYMMETRY In 1872, Felix Klein proposed the idea that geometry was best thought of as the study of invariants of a group of transformations. This had a profound effect on the study of geometry, eventually elevating symmetry to a central role. This thesis embodies the spirit of Klein's Erlangen program in the modern context of finite geometries { we employ knowledge about finite classical groups to solve long-standing problems in the area. We first look at hyperovals in finite Desarguesian projective planes. In the last 25 years a number of infinite families have been constructed. The area has seen a lot of activity, motivated by links with flocks, generalized quadrangles, and Laguerre planes, amongst others. An important element in the study of hyperovals and their related objects has been the determination of their groups { indeed often the only way of distinguishing them has been via such a calculation. We compute the automorphism group of the family of ovals constructed by Cherowitzo in 1998, and also obtain general results about groups acting on hyperovals, including a classification of hyperovals with large automorphism groups. We then turn our attention to finite Laguerre planes. We characterize the Miquelian Laguerre planes as those admitting a group containing a non-trivial elation and acting tran- sitively on flags, with an additional hypothesis { a quasiprimitive action on circles for planes of odd order, and insolubility of the group for planes of even order. -
Euclidean Versus Projective Geometry
Projective Geometry Projective Geometry Euclidean versus Projective Geometry n Euclidean geometry describes shapes “as they are” – Properties of objects that are unchanged by rigid motions » Lengths » Angles » Parallelism n Projective geometry describes objects “as they appear” – Lengths, angles, parallelism become “distorted” when we look at objects – Mathematical model for how images of the 3D world are formed. Projective Geometry Overview n Tools of algebraic geometry n Informal description of projective geometry in a plane n Descriptions of lines and points n Points at infinity and line at infinity n Projective transformations, projectivity matrix n Example of application n Special projectivities: affine transforms, similarities, Euclidean transforms n Cross-ratio invariance for points, lines, planes Projective Geometry Tools of Algebraic Geometry 1 n Plane passing through origin and perpendicular to vector n = (a,b,c) is locus of points x = ( x 1 , x 2 , x 3 ) such that n · x = 0 => a x1 + b x2 + c x3 = 0 n Plane through origin is completely defined by (a,b,c) x3 x = (x1, x2 , x3 ) x2 O x1 n = (a,b,c) Projective Geometry Tools of Algebraic Geometry 2 n A vector parallel to intersection of 2 planes ( a , b , c ) and (a',b',c') is obtained by cross-product (a'',b'',c'') = (a,b,c)´(a',b',c') (a'',b'',c'') O (a,b,c) (a',b',c') Projective Geometry Tools of Algebraic Geometry 3 n Plane passing through two points x and x’ is defined by (a,b,c) = x´ x' x = (x1, x2 , x3 ) x'= (x1 ', x2 ', x3 ') O (a,b,c) Projective Geometry Projective Geometry -

Proof with Parallelogram Vertices
Proof with Parallelogram Vertices About Illustrations: Illustrations of the Standards for Mathematical Practice (SMP) consist of several pieces, including a mathematics task, student dialogue, mathematical overview, teacher reflection questions, and student materials. While the primary use of Illustrations is for teacher learning about the SMP, some components may be used in the classroom with students. These include the mathematics task, student dialogue, and student materials. For additional Illustrations or to learn about a professional development curriculum centered around the use of Illustrations, please visit mathpractices.edc.org. About the Proof with Parallelogram Vertices Illustration: This Illustration’s student dialogue shows the conversation among three students who are trying to prove a conjecture they’ve made regarding the location of the possible fourth vertices of a parallelogram given only three vertices. As students try to prove their conjecture they struggle with proving the collinearity of points and begin intuitively using the parallel postulate. Highlighted Standard(s) for Mathematical Practice (MP) MP 1: Make sense of problems and persevere in solving them. MP 3: Construct viable arguments and critique reasoning of others. MP 6: Attend to precision. Target Grade Level: Grades 9–10 Target Content Domain: Congruence (Geometry Conceptual Category) Highlighted Standard(s) for Mathematical Content G-CO.C.9 Prove theorems about lines and angles. Theorems include: vertical angles are congruent; when a transversal crosses parallel lines, alternate interior angles are congruent and corresponding angles are congruent; points on a perpendicular bisector of a line segment are exactly those equidistant from the segment’s endpoints. G-CO.C.10 Prove theorems about triangles. -

Diagnostic Tools and Remedial Methods for Collinearity in Linear Regression Models with Spatially Varying Coefficients
DIAGNOSTIC TOOLS AND REMEDIAL METHODS FOR COLLINEARITY IN LINEAR REGRESSION MODELS WITH SPATIALLY VARYING COEFFICIENTS DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By David C. Wheeler, M.A.S., M.A. ***** The Ohio State University 2006 Dissertation Committee: Approved by Professor Morton O’Kelly, Advisor ____________________ Professor Catherine Calder, Advisor Advisor Graduate Program in Geography Professor Prem Goel Professor Ningchuan Xiao ABSTRACT The realization in the statistical and geographical sciences that a relationship between an explanatory variable and a response variable in a linear regression model is not always constant across a study area has lead to the development of regression models that allow for spatially varying coefficients. Two competing models of this type are geographically weighted regression (GWR) and Bayesian regression models with spatially varying coefficient processes (SVCP). In the application of these spatially varying coefficient models, marginal inference on the regression coefficient spatial processes is typically of primary interest. In light of this fact, there is a need to assess the validity of such marginal inferences, since these inferences may be misleading in the presence of explanatory variable collinearity. The presence of local collinearity in the absence of global collinearity necessitates the use of diagnostic tools in the local regression model building process to highlight areas in which the results are not reliable for statistical inference. The method of ridge regression and the lasso can also be integrated into the GWR framework to constrain and stabilize regression coefficients and lower prediction error. -

Concurrency and Collinearity in Hexagons
Journal for Geometry and Graphics Volume 20 (2016), No. 2, 159{171. Concurrency and Collinearity in Hexagons Nicolae Anghel Department of Mathematics, University of North Texas 1155 Union Circle #311430, Denton, TX 76203, USA email: [email protected] Abstract. In a cyclic hexagon the main diagonals are concurrent if and only if the product of three mutually non-consecutive sides equals the product of the other three sides. We present here a vast generalization of this result to (closed) hexagonal paths (Sine-Concurrency Theorem), which also admits a collinearity version (Sine-Collinearity Theorem). The two theorems easily produce a proof of Desargues' Theorem. Henceforth we recover all the known facts about Fermat- Torricelli points, Napoleon points, or Kiepert points, obtained in connection with erecting three new triangles on the sides of a given triangle and then joining appropriate vertices. We also infer trigonometric proofs for two classical hexagon results of Pascal and Brianchon. Key Words: Hexagon, Concurrency, Collinearity, Fermat-Torricelli Point, Napoleon Point, Kiepert Point, Desargues' Theorem, Pascal's Theorem, Brianchon's Theorem MSC 2010: 51M04, 51A05, 51N15, 97G70 1. Two Sine-Theorems Let A1A2A3A4A5A6 be a cyclic hexagon. A lesser known but nonetheless beautiful result states that the three main diagonals A1A4, A2A5, and A3A6 are concurrent if and only if A1A2 · A3A4 · A5A6 = A2A3 · A4A5 · A6A1 [4]. Is there an equivalent of this result, holding for non-cyclic convex hexagons? The answer is yes, and it turns out to be true in much greater generality, for hexagons not necessarily convex, and not even simple, when viewed as closed (polygonal) curves. -

Collinearity Varsity Practice 1/19/20 Tudor-Dimitrie Popescu
Western PA ARML January 19, 2020 Collinearity Varsity Practice 1/19/20 Tudor-Dimitrie Popescu 1 Warm-Up Problems 1. Revisit similarity. Read the proofs of Menelaus's and Ceva's theorems. 2. In a quadrilateral ABCD; let AD \ BC = Q; AB \ CD = P; AC \ BD = R; QR \ AB = K; P R \ BC = L; AC \ PQ = T . Prove that K,L and T are collinear. Hint: use Menelaus and Ceva 3. Let ABC be an acute triangle, and let M; N be distinct points on the segment BC such that \BAM = \CAN: If O1;O2 are the circumcenters of 4ABC and 4AMN respectively. Prove that A; O1;O2 are collinear. Hint: don't use Menelaus and Ceva; angle chasing goes a long way: you might want to prove that \O2AB = \O1AB: 2 Problem Set 1. (Monge) Let C1;C2;C3 be three circles and let their pairwise common tangents intersect at X; Y; Z: Prove that X; Y; Z are collinear. 2. (Simson) Given a point P in the plane of 4ABC. Suppose X; Y and Z are the feet of perpendiculars from P to BC; CA and AB respectively. Then X; Y and Z are collinear if and only if P lies on the circumcircle of 4ABC: 3. (Pappus) Let P1;P3;P5 and P2;P4;P6 be sets of collinear points (with P1P3 not parallel to P2P4). If P2P3 \ P5P6 = fM1g;P1P6 \ P3P4 = fM2g;P1P2 \ P4P5 = fM3g; show that M1M2;M3 are collinear. 4. (Desargues) Let A1B1C1 and A2B2C2 be triangles. IF fP g = B1C1 \ B2C2; fQg = A1C1 \ A2C2; fRg = A1B1 \ A2B2; then P; Q; R are collinear if and only if A1A2;B1B2;C1C2 meet at the same point.