Functional Anatomy of the Vertebral Vasculature in Reptiles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Anatomical Exploration Into the Variable Patterns of the Venous Vasculature of the Human Kidney
AN ANATOMICAL EXPLORATION INTO THE VARIABLE PATTERNS OF THE VENOUS VASCULATURE OF THE HUMAN KIDNEY. by KAPIL SEWSARAN SATYAPAL Submitted in partial fulfilment of the requirements for the degree of DOCTOR OF MEDICINE in the Department of Surgery University of Natal Durban 1993 To my wife Pratima, daughter Vedika, son Pravir, and my family. m ABSTRACT In clinical anatomy, the renal venous system is relatively understudied compared to the arterial system. This investigation aims to clarify and update the variable patterns of the renal venous vasculature using cadaveric human (adult and foetal) and Chacma baboon (Papio ursinus) kidneys and to reflect on its clinical application, particularly in surgery and radiology. The study employed gross anatomical dissection and detailed morphometric and statistical analyses on resin cast and plastinated kidneys harvested from 211 adult, 20 foetal and 10 baboon cadavers. Radiological techniques were used to study intrarenal flow, renal veins and collateral pathways and renal vein valves. The gross anatomical description of the renal veins and its relations were confirmed and updated. Additional renal veins were observed much more frequently on the right side (31 %) than previously documented (15.4%). A practical classification system for the renal veins based on the number of primary tributaries, additional renal veins and anomalies is proposed. Detailed morphometric analyses of the various parameters of the renal veins corroborated and augmented previous anatomical studies. Contrary to standard anatomical textbooks, it was noted that the left renal vein is 2.5 times the length of its counterpart and that there are variable levels of entry of the renal veins into the IVe. -

GROSS ANATOMY and CLINICAL PROBLEMS of CNS BLOOD SUPPLY and GLOSSOPHARYNGEAL NERVE © 2019Zillmusom
GROSS ANATOMY AND CLINICAL PROBLEMS OF CNS BLOOD SUPPLY AND GLOSSOPHARYNGEAL NERVE © 2019zillmusom I. OVERVIEW - Branches to CNS are described as arising from two sources: Vertebral and Internal Carotid arteries. A. Spinal Cord - Anterior Arteries and Posterior Spinal Arteries form as branches of Vertebral Arteries; however, most blood supply to the spinal cord is derived from Radicular arteries (branches of segmental arteries that enter via Intervertebral Foramina) B. Brain - Common Carotid arteries bifurcate to Internal and External Carotid arteries; Internal Carotid arteries supply 80% of brain; 15% of strokes are associated with stenosis (narrowing) of Internal Carotid artery at or near bifurcation. II. GROSS ANATOMY OF BLOOD SUPPLY OF SPINAL CORD A. Arterial supply 1. Anterior Spinal artery - single artery formed from branches of both Vertebral arteries; courses on anterior surface of cord. 2. Posterior Spinal arteries - paired arteries dorsolateral to spinal cord; arise (75%) from Posterior Inferior Cerebellar arteries (branch of Vertebral Artery) or directly from Vertebral arteries (25%). 3. Radicular (root) arteries - Most of blood supply to spinal cord is provided by Radicular (root) arteries; most these arteries arise from the Aorta and enter the spinal canal through Intervertebral foramina; one particularly large artery (Great Radicular Artery of Adamkiewicz, usually unpaired) arises from T9-T12 and provides major blood supply to lumbar and sacral spinal cord. Clinical Note: Obstruction of Radicular Artery (of Adamkiewicz) - Can occur during clamping for heart surgery or by a dissecting Aortic aneurysm; causes infarction (tissue death in spinal cord) similar to an Anterior Spinal Artery syndrome (symptoms include paraplegia (Corticospinal tracts, bilateral voluntary paralysis of legs and lower body), loss of pain and temperature sense (Spinothalamc tract, loss of sphincter control) with sparing of vibration and position sense (Dorsal Columns, sensory). -

Intervertebral Veins Directly Connecting the Vertebral Venous System to the Azygos Venous System Rather Than the Proximal End Of
Intervertebral Veins Rev Arg de Anat Clin; 2015, 7 (2): 88-92 ___________________________________________________________________________________________ Original Communication INTERVERTEBRAL VEINS DIRECTLY CONNECTING THE VERTEBRAL VENOUS SYSTEM TO THE AZYGOS VENOUS SYSTEM RATHER THAN THE PROXIMAL END OF THE POSTERIOR INTERCOSTAL VEINS Naief Dahran1,2, Roger Soames1 1Centre for Anatomy and Human Identification, College of Art, Science and Engineering, University of Dundee, Dundee, United Kingdom 2Department of Anatomy, College of Medicine, University of Jeddah, Jeddah, Kingdom of Saudi Arabia RESUMEN ABSTRACT La estructura de las venas de la cavidad torácica varía Veins in the thoracic cavity are highly variable in terms significativamente en función de sus conexiones. of their communications. Thirty Thiel-embalmed Treinta cadáveres embalsamados con la técnica de cadavers were dissected (18 females and 12 males), Thiel fueron disecados (18 mujeres, 12 hombres), con ranging in age from 48 to 98 years old (mean edades comprendidas entre 48 y 98 años (media 81.3±12.40). The lungs, heart, thoracic aorta, 81.3±12.40). Los pulmones, el corazón, la aorta oesophagus and parietal pleura were removed torácica, el esófago y la pleura parietal fueron carefully to expose the azygos, hemiazygos, accessory cuidadosamente retirados para permitir la visualización hemiazygos veins and thoracic duct. In most de las venas ácigos, hemiácigos y hemiácigos specimens (21) intervertebral veins were connected accesoria así como el conducto torácico. En la directly to the azygos venous systems rather than the mayoría de los especímenes (21) se encontró que las proximal end of the posterior intercostal veins. This venas intervertebrales estaban directamente presentation was observed to be more common on the conectadas con el sistema venoso ácigos en vez de right side, but not at all vertebral levels. -

An Experimental Study of the Venous Collateral Circulation of the Lung I
AN EXPERIMENTAL STUDY OF THE VENOUS COLLATERAL CIRCULATION OF THE LUNG I. ANATOMaCAL OBSERVATIONS * ADz.x HuRwnrz, M.D., Mso CAA , MD., RoNALD W. Coon:, MD., and AvErL A. Lsnow, MD. (From the Departments of Surgery and Medicin, Newington and West Havex Veterans Administration Hospitals, and Yale University Sckool of Medicine and tke Yal Departmext of Pathology, Newv Havex, Con.) Evidence has accrued recently of the etence of an extensive venous collateral circulation in some types of pulmonary disease in man, espe- cially in emphysema-" Quantitative estimates of the volume of the blood flow through these collaterals are difficult, if not impossible, to obtain in hulman subjects with methods currently available. This fact has prompted an attempt at the experimental production and func- tional evaluation of such a collateral circulation in the dog. The gen- eral pattern established in an earlier experimental investigation of bronchial arterial collateral circulation has been followed.' Although dogs could survive ligature of the venous drainage of a pulmonary lobe, it was long thought that this procedure was inevitably followed by hemorrhage, "atelectasis," and often by pneumonitis and thrombosis of the pulmonary veins.' The clinical observation that tuberculosis is rarely progressive in lungs which are the seat of chronic passive congestion, received experimental support from Tiegel,5 who constricted the pulmonary veins in dogs and rabbits. A similar opera- tion has since been used in the treatment of pulmonary tuberculosis in man.7 When penicillin came to be employed postoperatively, it was found possible to reduce strikingly the mortality following ligature of the veins of an entire lung.l°0,1 Although gross and histologic changes after this operation have been described in detail, especally by Wyatt and associates,11 there is little information regarding the collateral circulation. -

Venous Congestive Myelopathy Due to Chronic Inferior Vena Cava Thrombosis Treated with Endovascular Stenting: Case Report and Review of the Literature
Venous Congestive Myelopathy due to Chronic Inferior Vena Cava Thrombosis Treated with Endovascular Stenting: Case Report and Review of the Literature 1 2 3 4 Diego Z. Carvalho, MD , Joshua D. Hughes, MD , Greta B. Liebo, MD , Emily C. Bendel, MD , 4 1 Haraldur Bjarnason, MD , and James P. Klaas, MD 1Department of Neurology, Mayo Clinic, Rochester, MN, USA. 2Department of Neurosurgery, Mayo Clinic, Rochester, MN, USA 3Department of Neuroradiology, Mayo Clinic, Rochester, MN, USA 4Department of Vascular & Interventional Radiology, Mayo Clinic, Rochester, MN, USA Journal of Vascular and Interventional Neurology, Vol. 8 Abstract Authors have no conflict of interest to disclose. Objective—Impaired inferior vena cava (IVC) outflow can lead to collateralization of blood to the valve- less epidural venous plexus, causing epidural venous engorgement and venous congestion. Herein we describe a case of chronic IVC thrombosis presenting as venous congestive myelopathy treated with angio- plasty and endovascular stenting. The pathophysiological mechanisms of cord injury are hypothesized, and IVC stenting application is evaluated. Methods—Case report and review of the literature. Results—IVC outflow obstruction has only rarely been associated with neurologic dysfunction, with reports of lumbosacral nerve root compression in the cases of IVC agenesis, compression, or occlusion. Although endovascular angioplasty with stenting is emerging as a leading treatment option for chronic IVC thrombosis, its use to treat neurologic complications is limited to one case report for intractable sciatica. Our case is the first description of IVC thrombosis presenting with venous congestive myelopathy, and treated successfully with IVC stenting. Conclusion—Venous congestive myelopathy should be seen as a broader clinical condition, including not only typical dural arteriovenous fistulas, but also disorders of venous outflow. -

Radiographic Anatomy of the Intervertebral Cervical and Lumbar Foramina 691
Diagnostic and Interventional Imaging (2012) 93, 690—697 View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector CONTINUING EDUCATION PROGRAM: FOCUS. Radiographic anatomy of the intervertebral cervical and lumbar foramina (vessels and variants) ∗ X. Demondion , G. Lefebvre, O. Fisch, L. Vandenbussche, J. Cepparo, V. Balbi Service de radiologie musculosquelettique, CCIAL, laboratoire d’anatomie, faculté de médecine de Lille, hôpital Roger-Salengro, CHRU de Lille, rue Émile-Laine, 59037 Lille, France KEYWORDS Abstract The intervertebral foramen is an orifice located between any two adjacent verte- Spine; brae that allows communication between the spinal (or vertebral) canal and the extraspinal Spinal cord; region. Although the intervertebral foramina serve as the path traveled by spinal nerve roots, Vessels vascular structures, including some that play a role in vascularization of the spinal cord, take the same path. Knowledge of this vascularization and of the origin of the arteries feeding it is essential to all radiologists performing interventional procedures. The objective of this review is to survey the anatomy of the intervertebral foramina in the cervical and lumbar spines and of spinal cord vascularization. © 2012 Éditions françaises de radiologie. Published by Elsevier Masson SAS. All rights reserved. The intervertebral foramen is an orifice located between any two adjacent vertebrae that allows communication between the spinal (or vertebral) canal and the extraspinal region. Although the intervertebral foramina serve as the path traveled by spinal nerve roots, vascular structures, including some that play a role in vascularization of the spinal cord, take the same path. Knowledge of this vascularization and of the source of the arteries feeding the spinal cord is therefore essential to all radiologists performing interventional procedures in view of the iatrogenic risks they present. -

The Venous System of the Human Foetal Spinal Cord. Scanning Electron Microscope of Vascular Corrosion Casts J
Folia Morphol. Vol. 73, No. 2, pp. 139–142 DOI: 10.5603/FM.2014.0020 O R I G I N A L A R T I C L E Copyright © 2014 Via Medica ISSN 0015–5659 www.fm.viamedica.pl The venous system of the human foetal spinal cord. Scanning electron microscope of vascular corrosion casts J. Zawiliński1, K. Zagórska-Świeży1, J. Składzień2 1SEM Laboratory of the ENT Clinic, University Hospital in Krakow, Poland 2Department of Otorhinolaryngology, Jagiellonian University, Krakow, Poland [Received 11 October 2013; Accepted 25 November 2013] The investigation was carried out on 16 human foetal cadavers at the age of 17–23 weeks from the time of conception. The foetal vascular system was injected with the synthetic resin MERCOX CL-2R and analysed in scanning electron microscope. The vascular system of the foetal spinal cord was studied. The foetal vascular system was characterised by high variability concerning the number, course and localisation of blood vessels. It contained numerous anastomoses with the inter- nal spinal venous plexuses, which included anterior and posterior radicular veins. Large arteries running on the surface of the spinal cord are accompanied by the homoname veins. The venous system of the investigated foetuses was divided into 2 categories of veins: internal veins responsible for the drainage of blood from the central area, that is central and peripheral veins coming radially to the surface of the spinal cord and external veins, which form the venous system of the surface of the spinal cord. The venous system of the foetal spinal cord was also examined as to the presence of the valves. -

A Syllabus of Core Surgical Anatomy
A Syllabus of Core Surgical Anatomy Background In February 2010, it was agreed that the Anatomy Committee would undertake to develop a new generic examination for implementation in 2012 to assess anatomy for surgical trainees. Content Anatomical questions relate to: • clinical examination – surface anatomy, inspection, palpation, percussion, auscultation, pelvic examination, testing for peripheral nerve injuries, potential sites of spread of tumours (as determined by anatomy e.g. lymphatic drainage of the breast) • urethral catheterization • vascular access (arterial and venous, peripheral and central) • the airway: maintenance, access • chest drainage • imaging (plain radiographs, CT, MRI, US, contrast studies) • surgical access – open and minimally invasive • endoscopy (GI, arthroscopy etc) • peripheral nerve blocks • percutaneous liver biopsy • trauma (aligned to anatomy in EMST) • common anatomical complications of routine surgical procedures • principles of anatomy: terminology, anatomical position, planes, relationships in regional anatomy, movements, tissues, systems, and anatomical variation. Syllabus Essential (+++) • What an early SET 1 trainee (PGY 2-3 with general experience) should know. • Must recognise, understand and be able to explain. • These structures comprise core basic surgical anatomy and are essential in inter-specialty communication. • Lack of knowledge could jeapordise patient safety. • Includes all common and important anatomical characteristics of the structure: location, constituent parts, relations, blood supply and lymphatic drainage, innervation, course and distribution, when the structure is at risk, effects of injury, and common variants of clinical importance. Desirable (++) • Should be able to describe the basic anatomy/location of the structure, its function, major nerve and blood supply ± lymphatic drainage, and general relations. Non-core (+) • Not considered core knowledge but may be appropriate for specialty-specific anatomy. -

The Suboccipital Cavernous Sinus
The suboccipital cavernous sinus Kenan I. Arnautovic, M.D., Ossama Al-Mefty, M.D., T. Glenn Pait, M.D., Ali F. Krisht, M.D., and Muhammad M. Husain, M.D. Departments of Neurosurgery and Pathology, University of Arkansas for Medical Sciences, and Laboratory Service, Veterans Administration Medical Center, Little Rock, Arkansas The authors studied the microsurgical anatomy of the suboccipital region, concentrating on the third segment (V3) of the vertebral artery (VA), which extends from the transverse foramen of the axis to the dural penetration of the VA, paying particular attention to its loops, branches, supporting fibrous rings, adjacent nerves, and surrounding venous structures. Ten cadaver heads (20 sides) were fixed in formalin, their blood vessels were perfused with colored silicone rubber, and they were dissected under magnification. The authors subdivided the V3 into two parts, the horizontal (V3h) and the vertical (V3v), and studied the anatomical structures topographically, from the superficial to the deep tissues. In two additional specimens, serial histological sections were acquired through the V3 and its encircling elements to elucidate their cross-sectional anatomy. Measurements of surgically and clinically important features were obtained with the aid of an operating microscope. This study reveals an astonishing anatomical resemblance between the suboccipital complex and the cavernous sinus, as follows: venous cushioning; anatomical properties of the V3 and those of the petrouscavernous internal carotid artery (ICA), namely their loops, branches, supporting fibrous rings, and periarterial autonomic neural plexus; adjacent nerves; and skull base locations. Likewise, a review of the literature showed a related embryological development and functional and pathological features, as well as similar transitional patterns in the arterial walls of the V3 and the petrous-cavernous ICA. -
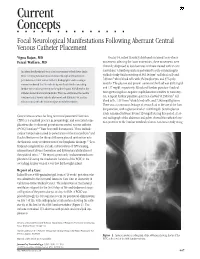
Current Concepts ⅢⅢⅢⅢⅢⅢⅢⅢⅢⅢⅢⅢⅢⅢ Focal Neurological Manifestations Following Aberrant Central Venous Catheter Placement
Current Concepts nnnnnnnnnnnnnn Focal Neurological Manifestations Following Aberrant Central Venous Catheter Placement Vigna Rajan, MD On day 14, infant B acutely developed sustained tonic-clonic Feizal Waffarn, MD movements affecting the lower extremities; these movements were clinically diagnosed as focal seizures and were treated with an anti- An infant developed focal tonic clonic movements of both lower limbs convulsant. A lumbar puncture performed to rule out meningitis 3 while receiving total parenteral nutrition through a left saphenous yielded cloudy fluid consisting of 34,114/mm red blood cells and 3 percutaneous central venous catheter. Radiographic studies using a 749/mm white blood cells with 3% lymphocytes and 97% poly- contrast confirmed that the catheter tip was located in the ascending morphs. The glucose and protein content of the fluid was 3943 mg/dl lumbar vein in close proximity to the epidural space. Withdrawal of the and 127 mg/dl, respectively. Blood and lumbar puncture fluid cul- catheter abated all clinical symptoms. This case emphasizes the need to tures grew coagulase-negative staphylococcus sensitive to vancomy- 3 confirm central venous catheter placement and illustrates yet another cin. A repeat lumbar puncture specimen showed 50,250/mm red 3 risk associated with the infusion of parenteral alimentation. blood cells, 1,515/mm white blood cells, and 7,348 mg/dl glucose. There was a continuous leakage of serous fluid at the site of the lum- bar puncture, with a glucose level of .800 mg/dl. Serum glucose levels remained between 80 and 120 mg/dl during this period. A lat- Central venous access for long-term total parenteral nutrition eral radiograph of the abdomen and pelvis showed the catheter loca- (TPN) is a standard practice in neonatology, and associated com- tion posterior to the lumbar vertebral column. -

The Development of the Vertebra and the Intervertebral Disc 1
1 THE DEVELOPMENT OF Disc Disease and Dynamic Stabilization Lumbar Degenerative THE VERTEBRA AND THE INTERVERTEBRAL DISC 1 Safiye CAVDAR M.D. fibrosus’. The nucleus pulposus’ plus the anulus fib- 1. Precartilage Stage (mesenchimal stage) rosus form the intervertebral disc. Some remnents of the notochord may remain within the interverteb- The vertebral column develops from the mesenchi- ral disc which can result as ‘chordoma’. This neop- mal cells that accumulated around the notochord lasm frequently occurs at the base of the skull and during the 4th week of the embryonic period. At the at lumbosacral region (1-3). end of the 4th week the mesenchimal cells that de- rive from the scleratom of the somits accumulates in 3 major regions (1-3). 1.b. Region surounding the neurol tube The mesenchimal cells at this region gives rise to 1.a. Region surounding the Notchord the vertebral archs. In the 4th week of the embryonic period the sclera- toms elgine around the notochord as paired mesenc- 1.c. Region surouunding the corpus hial cells. Each of the scleratoms cells are grouped lo- The mesenchimal cells at this region gives rise to the osely at cranial and compact at caudal levels. Some costal processes. The costal porocesses will give rise of the dense cells groups migrate cranially and form to the ribs at thoracic region (1-3). the intervertebral disc. The rest of the dens cell group together with the caudal loose scleratom group uni- 2. Cartilage Stage tes and forms the mesenchimal vertebral centrum. Each centrum is formed by two adjacent scleratom At the 6th week of the embryonal stage the mesenchi- and forms an intergemental structure. -

The Cerebrospinal Venous System: Anatomy, Physiology, and Clinical Implications Edward Tobinick, MD
5/8/2017 www.medscape.org/viewarticle/522597_print www.medscape.org The Cerebrospinal Venous System: Anatomy, Physiology, and Clinical Implications Edward Tobinick, MD Posted: 2/22/2006 Abstract and Introduction Abstract There is substantial anatomical and functional continuity between the veins, venous sinuses, and venous plexuses of the brain and the spine. The term "cerebrospinal venous system" (CSVS) is proposed to emphasize this continuity, which is further enhanced by the general lack of venous valves in this network. The first of the two main divisions of this system, the intracranial veins, includes the cortical veins, the dural sinuses, the cavernous sinuses, and the ophthalmic veins. The second main division, the vertebral venous system (VVS), includes the vertebral venous plexuses which course along the entire length of the spine. The intracranial veins richly anastomose with the VVS in the suboccipital region. Caudally, the CSVS freely communicates with the sacral and pelvic veins and the prostatic venous plexus. The CSVS constitutes a unique, largecapacity, valveless venous network in which flow is bidirectional. The CSVS plays important roles in the regulation of intracranial pressure with changes in posture, and in venous outflow from the brain. In addition, the CSVS provides a direct vascular route for the spread of tumor, infection, or emboli among its different components in either direction. Introduction "... we begin to wonder whether our conception of the circulation today is completely acceptable. As regards the venous part of the circulation, I believe our present conception is incorrect." Herlihy[1] "It seems incredible that a great functional complex of veins would escape recognition as a system until 1940..