Imaging in Spinal Posterior Epidural Space Lesions: a Pictorial Essay
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Anatomical Exploration Into the Variable Patterns of the Venous Vasculature of the Human Kidney
AN ANATOMICAL EXPLORATION INTO THE VARIABLE PATTERNS OF THE VENOUS VASCULATURE OF THE HUMAN KIDNEY. by KAPIL SEWSARAN SATYAPAL Submitted in partial fulfilment of the requirements for the degree of DOCTOR OF MEDICINE in the Department of Surgery University of Natal Durban 1993 To my wife Pratima, daughter Vedika, son Pravir, and my family. m ABSTRACT In clinical anatomy, the renal venous system is relatively understudied compared to the arterial system. This investigation aims to clarify and update the variable patterns of the renal venous vasculature using cadaveric human (adult and foetal) and Chacma baboon (Papio ursinus) kidneys and to reflect on its clinical application, particularly in surgery and radiology. The study employed gross anatomical dissection and detailed morphometric and statistical analyses on resin cast and plastinated kidneys harvested from 211 adult, 20 foetal and 10 baboon cadavers. Radiological techniques were used to study intrarenal flow, renal veins and collateral pathways and renal vein valves. The gross anatomical description of the renal veins and its relations were confirmed and updated. Additional renal veins were observed much more frequently on the right side (31 %) than previously documented (15.4%). A practical classification system for the renal veins based on the number of primary tributaries, additional renal veins and anomalies is proposed. Detailed morphometric analyses of the various parameters of the renal veins corroborated and augmented previous anatomical studies. Contrary to standard anatomical textbooks, it was noted that the left renal vein is 2.5 times the length of its counterpart and that there are variable levels of entry of the renal veins into the IVe. -

GROSS ANATOMY and CLINICAL PROBLEMS of CNS BLOOD SUPPLY and GLOSSOPHARYNGEAL NERVE © 2019Zillmusom
GROSS ANATOMY AND CLINICAL PROBLEMS OF CNS BLOOD SUPPLY AND GLOSSOPHARYNGEAL NERVE © 2019zillmusom I. OVERVIEW - Branches to CNS are described as arising from two sources: Vertebral and Internal Carotid arteries. A. Spinal Cord - Anterior Arteries and Posterior Spinal Arteries form as branches of Vertebral Arteries; however, most blood supply to the spinal cord is derived from Radicular arteries (branches of segmental arteries that enter via Intervertebral Foramina) B. Brain - Common Carotid arteries bifurcate to Internal and External Carotid arteries; Internal Carotid arteries supply 80% of brain; 15% of strokes are associated with stenosis (narrowing) of Internal Carotid artery at or near bifurcation. II. GROSS ANATOMY OF BLOOD SUPPLY OF SPINAL CORD A. Arterial supply 1. Anterior Spinal artery - single artery formed from branches of both Vertebral arteries; courses on anterior surface of cord. 2. Posterior Spinal arteries - paired arteries dorsolateral to spinal cord; arise (75%) from Posterior Inferior Cerebellar arteries (branch of Vertebral Artery) or directly from Vertebral arteries (25%). 3. Radicular (root) arteries - Most of blood supply to spinal cord is provided by Radicular (root) arteries; most these arteries arise from the Aorta and enter the spinal canal through Intervertebral foramina; one particularly large artery (Great Radicular Artery of Adamkiewicz, usually unpaired) arises from T9-T12 and provides major blood supply to lumbar and sacral spinal cord. Clinical Note: Obstruction of Radicular Artery (of Adamkiewicz) - Can occur during clamping for heart surgery or by a dissecting Aortic aneurysm; causes infarction (tissue death in spinal cord) similar to an Anterior Spinal Artery syndrome (symptoms include paraplegia (Corticospinal tracts, bilateral voluntary paralysis of legs and lower body), loss of pain and temperature sense (Spinothalamc tract, loss of sphincter control) with sparing of vibration and position sense (Dorsal Columns, sensory). -

Ligaments of the Costovertebral Joints Including Biomechanics, Innervations, and Clinical Applications: a Comprehensive Review W
Open Access Review Article DOI: 10.7759/cureus.874 Ligaments of the Costovertebral Joints including Biomechanics, Innervations, and Clinical Applications: A Comprehensive Review with Application to Approaches to the Thoracic Spine Erfanul Saker 1 , Rachel A. Graham 2 , Renee Nicholas 3 , Anthony V. D’Antoni 2 , Marios Loukas 1 , Rod J. Oskouian 4 , R. Shane Tubbs 5 1. Department of Anatomical Sciences, St. George's University School of Medicine, Grenada, West Indies 2. Department of Anatomy, The Sophie Davis School of Biomedical Education 3. Department of Physical Therapy, Samford University 4. Neurosurgery, Complex Spine, Swedish Neuroscience Institute 5. Neurosurgery, Seattle Science Foundation Corresponding author: Erfanul Saker, [email protected] Abstract Few studies have examined the costovertebral joint and its ligaments in detail. Therefore, the following review was performed to better elucidate their anatomy, function and involvement in pathology. Standard search engines were used to find studies concerning the costovertebral joints and ligaments. These often- overlooked ligaments of the body serve important functions in maintaining appropriate alignment between the ribs and spine. With an increasing interest in minimally invasive approaches to the thoracic spine and an improved understanding of the function and innervation of these ligaments, surgeons and clinicians should have a good working knowledge of these structures. Categories: Neurosurgery, Orthopedics, Rheumatology Keywords: costovertebral joint, spine, anatomy, thoracic Introduction And Background The costovertebral joint ligaments are relatively unknown and frequently overlooked anatomical structures [1]. Although small and short in size, they are abundant, comprising 108 costovertebral ligaments in the normal human thoracic spine, and they are essential to its stability and function [2-3]. -

Pain Pattern Explanation Forms
Pain Pattern Explanation Forms 1. Cervical Facet Pain Pattern 2. Cervical Radicular/Dynatome Pain Pattern 3. Costotransverse Joint Pain Pattern 4. Fibromyalgia Points 5. Hip Joint Pain Pattern 6. Lumbar Dermatomes: Chemical Radiculitis 7. Lumbar Dermatomes: Disc Pathology 8. Lumbar Disc Pathology Healed 9. Lumbar Epidural Fibrosis 10. Lumbar Facet Pain Pattern 11. Lumbar Stenosis 12. Sacroiliac Joint Pain Pattern 13. Thoracic Facet Pain Pattern 14. Upper Cervical Joint Pain Pattern The OEA pain pattern handouts are PDF files that can be used for patient education and marketing. They help you explain your diagnosis with original illustrations that the patients can take home with them. There is limited text so you can tell your explanation of the treatment plan. The pain patterns can be printed in color or black and white. Once purchased, our business card will be replaced with yours to personalize each handout. Each illustration is based on the pain patterns that have been established in books or research articles when available. Normal anatomy and pathoanatomy illustrations are shown for the clinician to explain the diagnosis to the patient and how their treatment can influence the pain generator. These can also be utilized as marketing tools. The following pages are some guidelines that can be utilized to explain the handouts to patients. Cervical Facet Pain Pattern The cervical facet joints are the joints of the neck. Neurophysiologic studies have shown that cervical facet‐joint capsules are sources of neck pain.1 Dwyer et al.2 established pain patterns of the cervical facet joints. o Parasagittal cervical and cervicothoracic pain. -

Canine Thoracic Costovertebral and Costotransverse Joints Three Case Reports of Dysfunction and Manual Therapy Guidelines for A
Topics in Compan An Med 29 (2014) 1–5 Topical review Canine Thoracic Costovertebral and Costotransverse Joints: Three Case Reports of Dysfunction and Manual Therapy Guidelines for Assessment and Treatment of These Structures Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physiotherapy), CAFCI, CCRTn Keywords: The costovertebral and costotransverse joints receive little attention in research. However, pain costovertebral associated with rib articulation dysfunction is reported to occur in human patients. The anatomic costotransverse structures of the canine rib joints and thoracic spine are similar to those of humans. As such, it is ribs physical therapy proposed that extrapolation from human physical therapy practice could be used for the assessment and rehabilitation treatment of the canine patient with presumed rib joint pain. This article presents 3 case studies that manual therapy demonstrate signs of rib dysfunction and successful treatment using primarily physical therapy manual techniques. General assessment and select treatment techniques are described. & 2014 Elsevier Inc. All rights reserved. The Canine Fitness Centre Ltd, Calgary, Alberta, Canada nAddress reprint requests to Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physiotherapy), CAFCI, CCRT, The Canine Fitness Centre Ltd, 509—42nd Ave SE, Calgary, Alberta, Canada T2G 1Y7 E-mail: [email protected] The articular structures of the thorax comprise facet joints, the erect spine and further presented that in reviewing the literature, intervertebral disc, and costal joints. Little research has been they were unable to find mention of natural development of conducted on these joints in human or animal medicine. However, idiopathic scoliosis in quadrupeds; however, there are reports of clinical case presentations in human journals, manual therapy avian models and adolescent models in man. -

Intervertebral Veins Directly Connecting the Vertebral Venous System to the Azygos Venous System Rather Than the Proximal End Of
Intervertebral Veins Rev Arg de Anat Clin; 2015, 7 (2): 88-92 ___________________________________________________________________________________________ Original Communication INTERVERTEBRAL VEINS DIRECTLY CONNECTING THE VERTEBRAL VENOUS SYSTEM TO THE AZYGOS VENOUS SYSTEM RATHER THAN THE PROXIMAL END OF THE POSTERIOR INTERCOSTAL VEINS Naief Dahran1,2, Roger Soames1 1Centre for Anatomy and Human Identification, College of Art, Science and Engineering, University of Dundee, Dundee, United Kingdom 2Department of Anatomy, College of Medicine, University of Jeddah, Jeddah, Kingdom of Saudi Arabia RESUMEN ABSTRACT La estructura de las venas de la cavidad torácica varía Veins in the thoracic cavity are highly variable in terms significativamente en función de sus conexiones. of their communications. Thirty Thiel-embalmed Treinta cadáveres embalsamados con la técnica de cadavers were dissected (18 females and 12 males), Thiel fueron disecados (18 mujeres, 12 hombres), con ranging in age from 48 to 98 years old (mean edades comprendidas entre 48 y 98 años (media 81.3±12.40). The lungs, heart, thoracic aorta, 81.3±12.40). Los pulmones, el corazón, la aorta oesophagus and parietal pleura were removed torácica, el esófago y la pleura parietal fueron carefully to expose the azygos, hemiazygos, accessory cuidadosamente retirados para permitir la visualización hemiazygos veins and thoracic duct. In most de las venas ácigos, hemiácigos y hemiácigos specimens (21) intervertebral veins were connected accesoria así como el conducto torácico. En la directly to the azygos venous systems rather than the mayoría de los especímenes (21) se encontró que las proximal end of the posterior intercostal veins. This venas intervertebrales estaban directamente presentation was observed to be more common on the conectadas con el sistema venoso ácigos en vez de right side, but not at all vertebral levels. -

Rib Mobilizations Kat Hayes, SPT
Rib Mobilizations Kat Hayes, SPT A) Anatomy a. 12 Thoracic Vertebrae i. Superior facet facets anteraior/lateral ii. Inferior facet facets posterior/medial iii. Plane of motion alows more lateral flexion but limited due to ribs iv. T2-10 have a superior and inferior demifacet where rib articulates with two vertebrae b. 12 Ribs i. Costovertebral facet 1. T2-10 rib head has an inferior and superior facet to articulates with 2 vertebrae 2. T1, T11-12 articulates with only one costal facet 3. Kinematics: gliding and rotation ii. Costotransverse Joint 1. Tubercle of rib articulates with on transverse processes 2. Kinematics: gliding wih some rotation c. Sternum i. Ribs 1-7 attach at sternum via costochondral joint ii. Ribs 8-10 articulate to costal cartilage iii. Ribs 11, 12 are not attached B) Young et al. mapped referral patterns for costotransverse joint patterns a. 8 asymptomatic male subjects b. Received consecutive, same day injections to either right T2,4,6 or to their left T3,5,7 c. OmnipaqueTM was injected using fluoroscopy into joint d. Subects were asked to desribe the pian using given descriptors and also VAS including description of referred pain C) Indications a. Rib or Thoracic hypomobility b. Pain c. Shallow breathing D) Contraindications a. Rib fracture b. Osteoperosis c. Hypermobility d. Malignancy e. Systemic inflamitory disease f. Ligamentous laxity E) Precautions a. Pulmonary Disease b. Severe Scoliosis c. Spinal fussion d. Pregnancy F) Assessment a. While patients sitting, assess breathing patern and palpate ribs for movement during inhilation/exhalation i. Are they a chest or belly breather ii. -

Disorders of the Thoracic Spine: Pathology and Treatment
Disorders of the thoracic spine: pathology and treatment CHAPTER CONTENTS • Extraspinal tumours involve the bony parts of the Disorders and their treatment e169 vertebrae. Benign tumours are usually located in the posterior parts (spinous and transverse processes), Tumours of the thoracic spine . e169 malignant tumours in the vertebral body. Extradural haematoma . e171 Spinal cord herniation . e173 Thoracic spinal canal stenosis . e173 Chest deformities . e173 Intraspinal tumours Fracture of a transverse process . e179 Spinal infections . e179 Thoracic neurofibroma Lateral recess stenosis . e180 Pathology Arthritis of the costovertebral and This benign tumour usually originates from the dorsal root and costotransverse joints . e180 arises from proliferating nerve fibres, fibroblasts and Schwann Arthritis of the thoracic facet joints . e181 cells. Sensory or motor fibres may be involved.1 Some confu- Paget’s disease . e181 sion exists about the terminology: various names, such as neu- rofibroma, neurinoma, neurilemmoma or schwannoma have been used. Some believe that all these terms cover the same type of tumour; others distinguish some slight histological dif- Disorders and their treatment ferences between them. Multiple tumours in nerve fibres and the subcutaneous tissues, often accompanied by patchy café Tumours of the thoracic spine au lait pigmentation, constitute the syndrome known as von Recklinghausen’s disease. Neuromas are the most common primary tumours of the Spinal neoplasms, both primary and secondary, are unusual spine accounting for approximately one-third of the cases. causes of thoracolumbar pain. However, because these lesions They are most often seen at the lower thoracic region and the are associated with high mortality, examiners must always be thoracolumbar junction.2 More than half of all these lesions are aware of the possibility of neoplastic diseases and must include intradural extramedullary (Fig 1), 25% are purely extradural, them in their differential diagnosis. -

Venous Congestive Myelopathy Due to Chronic Inferior Vena Cava Thrombosis Treated with Endovascular Stenting: Case Report and Review of the Literature
Venous Congestive Myelopathy due to Chronic Inferior Vena Cava Thrombosis Treated with Endovascular Stenting: Case Report and Review of the Literature 1 2 3 4 Diego Z. Carvalho, MD , Joshua D. Hughes, MD , Greta B. Liebo, MD , Emily C. Bendel, MD , 4 1 Haraldur Bjarnason, MD , and James P. Klaas, MD 1Department of Neurology, Mayo Clinic, Rochester, MN, USA. 2Department of Neurosurgery, Mayo Clinic, Rochester, MN, USA 3Department of Neuroradiology, Mayo Clinic, Rochester, MN, USA 4Department of Vascular & Interventional Radiology, Mayo Clinic, Rochester, MN, USA Journal of Vascular and Interventional Neurology, Vol. 8 Abstract Authors have no conflict of interest to disclose. Objective—Impaired inferior vena cava (IVC) outflow can lead to collateralization of blood to the valve- less epidural venous plexus, causing epidural venous engorgement and venous congestion. Herein we describe a case of chronic IVC thrombosis presenting as venous congestive myelopathy treated with angio- plasty and endovascular stenting. The pathophysiological mechanisms of cord injury are hypothesized, and IVC stenting application is evaluated. Methods—Case report and review of the literature. Results—IVC outflow obstruction has only rarely been associated with neurologic dysfunction, with reports of lumbosacral nerve root compression in the cases of IVC agenesis, compression, or occlusion. Although endovascular angioplasty with stenting is emerging as a leading treatment option for chronic IVC thrombosis, its use to treat neurologic complications is limited to one case report for intractable sciatica. Our case is the first description of IVC thrombosis presenting with venous congestive myelopathy, and treated successfully with IVC stenting. Conclusion—Venous congestive myelopathy should be seen as a broader clinical condition, including not only typical dural arteriovenous fistulas, but also disorders of venous outflow. -

Costovertebral and Costotransverse Joint Involvement in Rheumatoid Arthritis
Ann Rheum Dis: first published as 10.1136/ard.37.5.473 on 1 October 1978. Downloaded from Annals of the Rheumatic Diseases, 1978, 37, 473475 Costovertebral and costotransverse joint involvement in rheumatoid arthritis MICHAEL J. COHEN, JOAN EZEKIEL, AND ROBERT H. PERSELLIN From the Division ofRheumatology, Department ofMedicine, and the Department ofRadiology, The University of Texas Health Science Center at San Antonio, USA SUMMARY Lesions of the costovertebral (CV) and costotransverse (CT) joints are distinctly unusual in rheumatoid arthritis. The patient presented had dramatic changes in these joints with destruction, ankylosis, and bony overgrowth. This led to a moderate respiratory impairment and a distinctive radiological presentation. The costovertebral (CV) and costotransverse (CT) palpation near the medial borders of the scapulae. joints are diarthrodial joints containing hyaline Chest expansion measured at the fourth intercostal articular cartilage and lined with synovial membrane space was 1.5 cm. She had diffuse tenderness of and can be involved in inflammatory joint disease multiple peripheral joints. There was extensive (Goldthwait, 1940; Dihlman and Frik, 1968; atrophy of the muscles of the hands. Erythema and Zimmer, 1968; Jaffe, 1972). However, lesions of the tenderness were detected over the right achilles copyright. ribs and their articulations with the vertebral column tendon. There were no rheumatoid nodules. are rare. The following is a case with unique involve- The haematocrit was 38 with a white blood cell ment of the CV and CT joints which has not been count of 4900/mm3. The erythrocyte sedimentation previously described. rate was 50 mm/hr (Westergren); a sensitised sheep cell agglutination titre was positive at 1:3584, and an Case report RA slide latex (Hyland) was 4+ positive (Cheng and Persellin, 1971). -

A Syllabus of Core Surgical Anatomy
A Syllabus of Core Surgical Anatomy Background In February 2010, it was agreed that the Anatomy Committee would undertake to develop a new generic examination for implementation in 2012 to assess anatomy for surgical trainees. Content Anatomical questions relate to: • clinical examination – surface anatomy, inspection, palpation, percussion, auscultation, pelvic examination, testing for peripheral nerve injuries, potential sites of spread of tumours (as determined by anatomy e.g. lymphatic drainage of the breast) • urethral catheterization • vascular access (arterial and venous, peripheral and central) • the airway: maintenance, access • chest drainage • imaging (plain radiographs, CT, MRI, US, contrast studies) • surgical access – open and minimally invasive • endoscopy (GI, arthroscopy etc) • peripheral nerve blocks • percutaneous liver biopsy • trauma (aligned to anatomy in EMST) • common anatomical complications of routine surgical procedures • principles of anatomy: terminology, anatomical position, planes, relationships in regional anatomy, movements, tissues, systems, and anatomical variation. Syllabus Essential (+++) • What an early SET 1 trainee (PGY 2-3 with general experience) should know. • Must recognise, understand and be able to explain. • These structures comprise core basic surgical anatomy and are essential in inter-specialty communication. • Lack of knowledge could jeapordise patient safety. • Includes all common and important anatomical characteristics of the structure: location, constituent parts, relations, blood supply and lymphatic drainage, innervation, course and distribution, when the structure is at risk, effects of injury, and common variants of clinical importance. Desirable (++) • Should be able to describe the basic anatomy/location of the structure, its function, major nerve and blood supply ± lymphatic drainage, and general relations. Non-core (+) • Not considered core knowledge but may be appropriate for specialty-specific anatomy. -
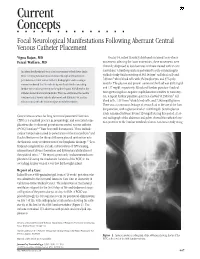
Current Concepts ⅢⅢⅢⅢⅢⅢⅢⅢⅢⅢⅢⅢⅢⅢ Focal Neurological Manifestations Following Aberrant Central Venous Catheter Placement
Current Concepts nnnnnnnnnnnnnn Focal Neurological Manifestations Following Aberrant Central Venous Catheter Placement Vigna Rajan, MD On day 14, infant B acutely developed sustained tonic-clonic Feizal Waffarn, MD movements affecting the lower extremities; these movements were clinically diagnosed as focal seizures and were treated with an anti- An infant developed focal tonic clonic movements of both lower limbs convulsant. A lumbar puncture performed to rule out meningitis 3 while receiving total parenteral nutrition through a left saphenous yielded cloudy fluid consisting of 34,114/mm red blood cells and 3 percutaneous central venous catheter. Radiographic studies using a 749/mm white blood cells with 3% lymphocytes and 97% poly- contrast confirmed that the catheter tip was located in the ascending morphs. The glucose and protein content of the fluid was 3943 mg/dl lumbar vein in close proximity to the epidural space. Withdrawal of the and 127 mg/dl, respectively. Blood and lumbar puncture fluid cul- catheter abated all clinical symptoms. This case emphasizes the need to tures grew coagulase-negative staphylococcus sensitive to vancomy- 3 confirm central venous catheter placement and illustrates yet another cin. A repeat lumbar puncture specimen showed 50,250/mm red 3 risk associated with the infusion of parenteral alimentation. blood cells, 1,515/mm white blood cells, and 7,348 mg/dl glucose. There was a continuous leakage of serous fluid at the site of the lum- bar puncture, with a glucose level of .800 mg/dl. Serum glucose levels remained between 80 and 120 mg/dl during this period. A lat- Central venous access for long-term total parenteral nutrition eral radiograph of the abdomen and pelvis showed the catheter loca- (TPN) is a standard practice in neonatology, and associated com- tion posterior to the lumbar vertebral column.