Geomembrane Lifetime Prediction: Geosynthetic Institute
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alternative Bottom Liner System
Engineering Report: Appendix C Volume 2 Alternative Bottom Liner System COWLITZ COUNTY HEADQUARTERS LANDFILL PROJECT COWLITZ COUNTY, WASHINGTON Alternative Bottom Liner System COWLITZ COUNTY HEADQUARTERS LANDFILL PROJECT COWLITZ COUNTY, WASHINGTON Prepared for COWLITZ COUNTY DEPARTMENT OF PUBLIC WORKS November 2012 Prepared by Thiel Engineering P.O. Box 1010 Oregon House, CA 95962 Table of Contents 1 INTRODUCTION ................................................................................................................... 1 1.1 Purpose and Scope ......................................................................................................................... 1 1.2 Background .................................................................................................................................... 1 1.3 Proposed Alternative ..................................................................................................................... 2 1.4 Description of GCLs ...................................................................................................................... 3 2 TECHNICAL EQUIVALENCY AND PERFORMANCE ................................................. 5 2.1 The Theory of Composite Liners with Reference to GCLs ....................................................... 5 2.2 Technical Equivalency Issues ....................................................................................................... 6 2.3 Hydraulic Issues ........................................................................................................................... -

Interface Friction Performance
CONTENTS Acknowledgements 1.0 Introduction 1.1 PVC and HDPE 2.0 Testing Program 2.1 Materials 2.1.1 Geomembranes 2.1.2 Soil 2.1.2.1 Sand 2.1.2.2 Sandy Loam 2.1.2.3 Silty Clay 2.1.3 Geotextile 2.2 Equipment 2.3 Procedure 3.0 Results 3.1 Sand vs Smooth PVC 3.2 Sand vs the Other geomembranes 3.3 Influence of Soil type 3.4 Geomembrane vs Geotextile 4.0 Summary of Results 12 5.0 Discussion 5.1 Failure Modes 5.2 General Observations 5.3 Comparison with existing knowledge 6.0 Conclusions 2 1 References 2 1 APPENDIX A 23 APPENDIX B 29 APPENDIX C 35 APPENDIX D 41 FIGURES Typical cross-sections of modem landfills Schematic representation of stress-strain behaviour of HDPE & PVC Grain-size distribution of Sand, Sandy Loam and Silty clay Reproducibility of test data Sand vs Smooth PVC : Test results Sand vs Smooth HDPE Sandy Loam vs Smooth PVC Silty Clay vs Smooth PVC Non-woven Geotextile vs Smooth PVC Appendix A : Fine Sand vs various Geomembranes Al. Sand vs Smooth PVC A2. Sand vs Textured PVC A3. Sand vs File-finish PVC A4. Sand vs Smooth HDPE A5. Sand vs Textured HDPE Appendix B : Sandy Loam vs various Geomembranes B 1. Sandy Loam vs Smooth PVC B2. Sandy Loam vs Textured PVC B3. Sandy Loam vs File-finish PVC B4. Sandy Loam vs Smooth-HDPE B5. Sandy Loam vs Textured HDPE Appendix C : Silty Clay vs various Geomembranes C1. Silty Clay vs Smooth PVC C2. -

Guidance on the Design and Construction of Leak-Resistant Geomembrane Boots and Attachments to Structures
Guidance on the Design and Construction of Leak-Resistant Geomembrane Boots and Attachments to Structures R. Thiel, Vector Engineering, Grass Valley, CA, USA G. DeJarnett, Envirocon, Houston, TX, USA ABSTRACT Experience in reviewing, designing, performing field inspections, and installing of geomembrane boots and connections to structures has revealed a widely diverse practice of standards and approaches. The execution of these details is very much an art in workmanship, and depends a great deal on the experience and understanding of the installer. There is very little guidance in the literature regarding the fine points of specifying and installing these critical details. The typical manufacturers’ details and guidelines are not much more than concepts that have been repeated for two decades. Thus, there is a big difference between what we assume and expect versus what is constructed in terms of leak resistance of geomembrane penetrations and attachments to structures. The goal of this paper is to touch upon some of the detailed and critical aspects that should be addressed when specifying and constructing geomembrane seals around penetrating pipes (referred to as “boots”) and attachments to structures. 1. INTRODUCTION While much attention has been paid in the last 30 years to many other containment issues related to geomembranes (such as chemical compatibility, aging and durability, manufacturing, seaming, subgrade preparation, covering), there is surprisingly little technical discussion related to the design and construction of leak-resistant penetrations and attachments to structures. This subject has largely been relegated to a few simple details, mostly generated by the manufacturers and included in their standard literature. The content of this paper is derived from the authors’ field observations, experience, and deductive reasoning. -

Geomembrane Puncture Potential and Hydraulic Performance in Mining Applications
GEOMEMBRANE PUNCTURE POTENTIAL AND HYDRAULIC PERFORMANCE IN MINING APPLICATIONS Lining systems in mining applications often consist of a geomembrane underlain by either a soil liner or a geosynthetic clay liner (GCL). When under load, geomembranes are vulnerable to damage from large stones both in the compacted soil subgrade and in the overlying drainage layer. Although guidance has been developed for minimizing geomembrane puncture, this past work has focused on subgrade protrusions in municipal solid waste applications. There has been limited information regarding puncture performance in mining applications, where extreme loads are encountered and angular, large-diameter crushed ore is often used as the drainage medium above the geomembrane. The attached paper discusses a laboratory puncture testing program involving various geomembranes placed in direct contact with different drainage media under high loads, both with and without underlying GCLs. Variables being examined include: geomembrane type and thickness, GCL type, normal load, and drainage stone size. Preliminary test results have shown that geomembrane/GCL composite liners are subject to less puncture damage (i.e., lower defect frequency and/or smaller puncture sizes) than geomembrane liners alone. The paper also presents a feasibility study of two lining alternatives, geomembrane/compacted soil and geomembrane/GCL composites. The feasibility study compares technical effectiveness and cost effectiveness based on cost savings associated with improved metal recovery rates afforded by improved containment. This information is intended for mining companies and engineers in evaluating lining options and allowable stone sizes. This paper was presented at the Tailings and Mine Waste ’08 Conference. TR 260 11/08 800.527.9948 Fax 847.577.5566 For the most up-to-date product information, please visit our website, www.cetco.com. -

Water Content–Density Criteria for Determining Geomembrane–Fly Ash Interface Shear Strength
MATEC Web of Conferences 262, 04005 (2019) https://doi.org/10.1051/matecconf/201926204005 KRYNICA 2018 Water Content–Density Criteria for Determining Geomembrane–Fly Ash Interface Shear Strength Katarzyna Zabielska-Adamska1,* 1 Bialystok Technical University, Faculty of Civil and Environmental Engineering, Wiejska Street 45E, 15-351 Bialystok, Poland Abstract. The aim of the present paper was to determine shear strength at the interface between fly ash, as a material underlying artificial sealing layer of storage yards, and HDPE geomembranes. The fly ash was compacted at moisture contents ranging over optimum water contents ± 5% using the standard Proctor test. The shear strength and interaction tests were conducted in classic direct shear apparatus with a cylindrical shear box. For interface strength tests the bottom box frame was equipped with a polycarbonate platen, which enabled geomembrane fixing. The shear strength of the interface contact of fly ash–smooth HDPE geomembrane did not greatly depend on moisture at compaction; however, it was important for textured geomembrane. The lowest interface strength was obtained at the highest moisture w= wopt + 5%, and the greatest values at moistures w ≥ wopt, for both geomembranes. 1 Introduction of compacted non-cohesive fly ash and fly ash/bottom ash mixture are dependent on moisture content during Mineral soil liners and covers are most often single, compaction, w, as are properties of cohesive mineral double or multilayer complex sealing, consisting of soils [14]. Consequently, different values are obtained compacted cohesive soil layers, with coefficient of for w on either side of the wopt on a compaction curve, –9 permeability, k, lower than 10 m/s, characterised by a for the same dry densities, ρd. -

Geomembranes and Seams
Geomembranes and Seams Ian D. Peggs I-CORP INTERNATIONAL, Inc., USA [email protected] ABSTRACT: Geomembranes and their seams have been destructively tested the same way for many years. However, it is still not appreciated that only shear elongation and peel separation provide useful information on weld integrity. It would be desirable to preclude seam destructive testing and to replace it with nondestructive testing (NDT) methods. Electrical methods are now available for locating leaks anywhere in liners whether covered or exposed. On landfill caps infrared spectroscopy can locate leaks much faster. Unfortunately, these methods will not assess the bond efficiency of a weld. However, ultrasonic (UT) methods, both pulse-echo and pitch-catch techniques are being developed to evaluate bond quality and the presence of internal flaws that may become leaks in service. The most promising method for the NDT evaluation of seam bond strength is infrared thermography (IRT) that even appears capable of identifying variations in weld zone microstructure due to cycling of the welder wedge temperature. 1 INTRODUCTION Geomembrane seams have been nondestructively and destructively tested the same way for many years. Only the acceptance criteria for destructively tested samples have been updated, but not far enough. However, seams are only a very small fraction of the total area of the liner which, until the development of electrical methods, could only be monitored visually It is past time that these test methods were reviewed and updated to take advantage of new technologies and the statistics generated over many years of testing. If this is not done we are wasting much time and are not achieving the highest quality lining systems. -

GEOMEMBRANE OR GEOSYNTHETIC CLAY LINER Code 521A (Ft2)
PA521-1 CONSERVATION PRACTICE STANDARD POND SEALING OR LINING - GEOMEMBRANE OR GEOSYNTHETIC CLAY LINER Code 521A (Ft2) DEFINITION A liner for an impoundment constructed using a geomembrane or a geosynthetic clay material. PURPOSE This practice is to: Reduce seepage losses from an impoundment for water conservation. Protect soil and water from contaminants. CONDITIONS WHERE PRACTICE APPLIES This practice applies where in-place natural soils have excessive seepage rates. CRITERIA General Criteria Applicable to Purposes. Design. The structure to be lined must meet all applicable NRCS standards. All inlets, outlets, ramps, and other appurtenances may be installed before, during, or after the liner placement, but must be done in a manner that does not damage or impair the proper operation of the liner. Flexible membrane liners must be planned, designed, and installed in conformance with all applicable federal, state, and local laws and regulations. Design and install the liner in accordance with manufacturer recommendations. The installer or manufacturer must certify that the liner installation meets the material and installation requirements of the plans and specifications. Follow manufacturer’s recommendations with regard to protection from weather and ultraviolet exposure. Materials. Geomembrane materials must meet the criteria in NRCS National Engineering Handbook (NEH), Part 642, Chapter 3, “Material Specification 594 – Geomembrane Liner.” GCL materials must meet the criteria in NRCS NEH, Part 642, Chapter 3, “Material Specification -
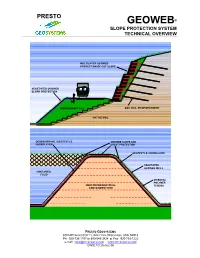
The Geoweb Slope Protection System Technical Overview
PRESTO GEOWEB® SLOPE PROTECTION SYSTEM TECHNICAL OVERVIEW MULTILAYER GEOWEB PROTECTION OF CUT SLOPE VEGETATED GEOWEB SLOPE PROTECTION EMBANKMENT FILL SOIL NAIL REINFORCEMENT NATIVE SOIL GEOMEMBRANE / GEOTEXTILE GEOWEB SLOPE AND UNDERLAYER CREST PROTECTION GEOTEXTILE UNDERLAYER VEGETATED GEOWEB INFILL CONTAINED FLUID INTEGRAL POLYMER REINFORCED EARTHFILL TENDON CONTAINMENT DIKE PRESTO GEOSYSTEMS 670 N PERKINS STREET, APPLETON, WISCONSIN, USA 54914 Ph: 920-738-1707 or 800-548-3424 ■ Fax: 920-738-1222 e-mail: [email protected] WWW.PRESTOGEO.COM/ GWSLTO 25-AUG-08 PRESTO ® GEOWEB SLOPE PROTECTION SYSTEM TECHNICAL OVERVIEW Table of Contents Introduction....................................................................................................................................................1 Examples of Geoweb Slope Surface Stabilization........................................................................................1 Surface Instability - Identifying Problems and Defining Causes ...................................................................1 General Surface Erosion Problems...........................................................................................................1 Localized Surface Instability Problems......................................................................................................1 General Slope Cover Instability Problems.................................................................................................2 Geoweb Slope Stabilization Systems - The Key Components .....................................................................2 -

Interface Friction of Smooth Geomembranes and Ottawa Sand
61 INFO TEKNIK, Volume 12 No. 1, Juli 2011 INTERFACE FRICTION OF SMOOTH GEOMEMBRANES AND OTTAWA SAND Rustam Effendi¹) Abstract ± Geomembranes commonly used in civil engineering constructions are mostly in contact with soils. Some constructions failed due to slippage between geomembrane sheets and interfacing soils. This paper aims at presenting the interface strength of various geomembranes and Ottawa sand resulting from tests with the ring shear device. The interface strength is generally governed by the stiffness, the texture of geomembranes and the imposed stress level. It was found that residual friction angles, dresidual, for the interfaces varied from 10.5° to 28.1° or 0.34 to 0.97 in efficiency ratio. The lower value is for a smooth HDPE, the higher value is mobilised by a soft PVC at higher stresses. Keywords: Interface, Geomembranes, Ottawa Sand BACKGROUND from the ring shear device, the shear displacement from the direct shear test is The uses of geomembranes, very limited. Often, the residual tails of the geosynthetic materials, have been common strength-horizontal displacement relation- in civil engineering constructions (Koerner ship are not apparent. In this research, the 1990; Sarsby 2007). In the applications, the ring shear device was deployed in order to materials are mostly in contact with soils. In simulate the field condition where the designs, however, the interface friction residual strength is reached at large behaviouris often forgotten to consider. This displacement. The Ottawa sand was used as led to failures of several constructions, for an interfacing soil. instance the slippage of landfill facility at the Kettleman Hills, California (Seed 1988). -

GEC 11 Design and Construction of Mechanically Stabilized Earth Walls and Reinforced Soil Slopes
U. S. Department of Transportation Publication No. FHWA-NHI-10-024 Federal Highway Administration FHWA GEC 011 – Volume I November 2009 NHI Courses No. 132042 and 132043 Design and Construction of Mechanically Stabilized Earth Walls and Reinforced Soil Slopes – Volume I Developed following: AASHTO LRFD Bridge Design and AASHTO LRFD Bridge Construction Specifications, 4th Edition, 2007, Specifications, 2nd Edition, 2004, with with 2008 and 2009 Interims. 2006, 2007, 2008, and 2009 Interims. NOTICE The contents of this report reflect the views of the authors, who are responsible for the facts and accuracy of the data presented herein. The contents do not necessarily reflect policy of the Department of Transportation. This report does not constitute a standard, specification, or regulation. The United States Government does not endorse products or manufacturers. Trade or manufacturer's names appear herein only because they are considered essential to the object of this document. Technical Report Documentation Page 1. REPORT NO. 2. GOVERNMENT 3. RECIPIENT'S CATALOG NO. ACCESSION NO. FHWA-NHI-10-024 FHWA GEC 011-Vol I 4. TITLE AND SUBTITLE 5. REPORT DATE November 2009 Design of Mechanically Stabilized Earth Walls 6. PERFORMING ORGANIZATION CODE and Reinforced Soil Slopes – Volume I 7. AUTHOR(S) 8. PERFORMING ORGANIZATION REPORT NO. Ryan R. Berg, P.E.; Barry R. Christopher, Ph.D., P.E. and Naresh C. Samtani, Ph.D., P.E. 9. PERFORMING ORGANIZATION NAME AND ADDRESS 10. WORK UNIT NO. Ryan R. Berg & Associates, Inc. 11. CONTRACT OR GRANT NO. 2190 Leyland Alcove DTFH61-06-D-00019/T-06-001 Woodbury, MN 55125 12. -

Using Geomembrane Liners to Reduce Seepage Through the Base of Tailings Ponds—A Review and a Framework for Design Guidelines
geosciences Review Using Geomembrane Liners to Reduce Seepage through the Base of Tailings Ponds—A Review and a Framework for Design Guidelines Anne Tuomela 1,* , Anna-Kaisa Ronkanen 2 , Pekka M. Rossi 2, Anssi Rauhala 1 , Harri Haapasalo 3 and Kauko Kujala 2 1 Structures and Construction Technology, University of Oulu, P.O. Box 4300, 90014 Oulu, Finland; anssi.rauhala@oulu.fi 2 Water, Energy and Environmental Engineering, University of Oulu, P.O. Box 4300, 90014 Oulu, Finland; anna-kaisa.ronkanen@oulu.fi (A.-K.R.); pekka.rossi@oulu.fi (P.M.R.); [email protected].fi (K.K.) 3 Industrial Engineering and Management, University of Oulu, P.O. Box 4300, 90014 Oulu, Finland; harri.haapasalo@oulu.fi * Correspondence: anne.tuomela@oulu.fi; Tel.: +358-50342-6151 Abstract: Geomembranes are used worldwide as basin liners in tailings ponds to decrease the permeability of the foundation and prevent further transportation of harmful contaminants and contaminated water. However, leakage into the environment and damage to the geomembrane have been reported. This paper reviews available literature and recommendations on geomembrane structures for use as a basal liner in tailings ponds, and presents a framework to achieve early Citation: Tuomela, A.; Ronkanen, involvement and an integrated approach to geomembrane structure design. Cohesive planning A.-K.; Rossi, P.M.; Rauhala, A.; guidelines or legislative directions for such structures are currently lacking in many countries, which Haapasalo, H.; Kujala, K. Using often means that the structure guidelines for groundwater protection or landfill are applied when Geomembrane Liners to Reduce designing tailings storage facilities (TSF). Basin structure is generally unique to each mine but, based Seepage through the Base of Tailings on the literature, in the majority of cases the structure has a single-composite liner. -

Microspike® Textured Geomembrane
MicroSpike® Textured Geomembrane Applications for HDPE and LLDPE MicroSpike, textured geomembranes include projects where slope stability is critical. MicroSpike is the only HDPE or LLDPE geomembrane that exhibits reproducible texture and friction angle values with the highest interface surface friction values in the industry. MicroSpike is a patented product. Agru America’s structured Geomembranes are produced on state-of-the-art equipment using a flat die-cast extrusion manufacturing process as opposed to blown film extrusion. Agru America is the only manufacturer of structured and embossed Geomembranes in North America. The flat die produces the most consistent geomembrane whether smooth or textured. MicroSpike surface texture Product Supply Range • Available in thicknesses of 30 -100 mil • Available in HDPE or LLDPE • Available in black/white, green and natural • Available in single- or double-sided US Patent - No. 6,203,741 and 6,132,845 Comparative properties for Design Consideration Blown film co-extruded textured surfaces vs. MicroSpike structured textured surfaces Design Consideration Blown film co-extruded MicroSpike structured Consistent core thickness no yes Consistent surface texture no yes Consistent asperity height no yes Consistent interface friction no yes Affect on mechanical properties yes no Affect on stress crack potential yes no Reduction in CQA program costs no yes (less testing required) The calendared structured liner manufacturing process allows production of the only textured liner with a consistent core thickness