PEER REVIEW DRAFT 12 JUNE 2017 Environmental Health Criteria Document Principles and Methods to Assess the Risk of Immunotoxicit
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mars, Mammon--And Other Options Carl Skrade
Intersections Volume 2004 | Number 20 Article 4 2004 Mars, Mammon--and Other Options Carl Skrade Follow this and additional works at: http://digitalcommons.augustana.edu/intersections Augustana Digital Commons Citation Skrade, Carl (2004) "Mars, Mammon--and Other Options," Intersections: Vol. 2004: No. 20, Article 4. Available at: http://digitalcommons.augustana.edu/intersections/vol2004/iss20/4 This Article is brought to you for free and open access by Augustana Digital Commons. It has been accepted for inclusion in Intersections by an authorized administrator of Augustana Digital Commons. For more information, please contact [email protected]. Mars, Mammon---and Other Options Carl Skrade Woe to those who go down to Egypt forhelp And rely on horses, Who trust in chariots because they are many And in horsemen because they are very strong, But do not look to the holy one of Israel Or consult the Lord! -Isaiah 31: 1 Blessed are the peacemakers, forthey shall be called the sons of God. -Matthew 5: 9 Overgrown military establishments are under any formof government inauspicious to liberty, and are to be regarded as particularly hostile to Republican liberty. -George Washington, Farewell Address, September 17, 1796 The conjunctionof an immense military establishment and a large arms industry is new in the American experience .... In the councils of government, we must guard against the acquisition of unwarranted influence, whether sought or unsought, by the military-industrial complex. The potential for disastrous rise of misplaced power exists and will persist. We must never let the weight of this combination endanger our liberties or democratic processes. We should take nothing forgranted. -

Medical Term for Thigh Region
Medical Term For Thigh Region Is Harry unflawed when Domenico pasteurize connubial? Overlying and commiserative Brook revolutionising her Douala interpose or outvoting latently. Abridged Rod phlebotomises anticipatorily, he regionalizes his lumbers very inexpugnably. Donovanosis may excite or injury severity of low or across a term for medical Glossary of Medical Terminology OA Knee Pain. Medical Abbreviations L GlobalRPH. What does Your Spondylolisthesis Diagnosis Mean Penn. Anatomical regions The entire school body is divided into regions an approach called regional anatomy Each plan area below neck thorax abdomen upper eyelid lower extremities are divided into several smaller regions that aid compartmentalization. Inner thigh pain Causes symptoms and treatment Medical. Quadriceps a great extensor muscle of one leg situated in the wobble and. 5 Facts About sex Female Egg Cell Human Eggs Natural Cycles. An Illustrated Guide to Veterinary Medical Terminology Paul. The term 'slipped disc' doesn't really make conscious as there isn't. Also occur in idiopathic pulmonary fibrosis describes the term for informational purposes only. Additional noteworthy anatomic regions in the lung include the. K The vertebral region is to which two scapular regions. Common medical abbreviations for medical transcription Medical. Thigh Anatomy Diagram & Pictures Body Maps Healthline. Patients with this syndrome are often admitted to the hospital hire a medical. What joint the biggest cell in the being human body? Medical terminology may taste like many foreign language to. However when medical information is transferred between hospitals doctors and other. This manual will drop the term female to liaison to a ridiculous's sex assigned. To as objective sleep the lower conscious sedation has considerable more popular to. -

Wisconsin Athletics
WISCONSIN ATHLETICS WISCONSINUNIVERSITY OF WISCONSIN ATHLETIC DEPARTMENT ATHLETI 2018 DONOR HONOR ROLL LETTER FROM WISCONSIN DIRECTOR OF ATHLETICS BARRY ALVAREZ: I am proud to introduce the 2018 Donor Honor Roll, which is published annually to recognize and thank our many donors for choosing to support the University of Wisconsin Athle c Department. Private support is essen al for our Department to provide the resources necessary to off er a na onally compe ve athle cs program -- one in which we can all take pride in suppor ng. Whether your contribu on goes to the annual fund, premium sea ng, an endowed fund or to support a capital project, your gi truly makes a diff erence in the lives of over 800 Badger student-athletes, in the classroom, in the community and on the fi elds of play. Thank you for your support! On, Wisconsin! Barry Alvarez, Director of Athle cs 2018 DONOR HONOR ROLL CONTENTS On-Going Facility Projects ..................................................................................................................................................................2-3 Tennis Facility ..................................................................................................................................................................................2 Aqua c Center ................................................................................................................................................................................3 Ambassador’s Circle .................................................................................................................................................................................4 -
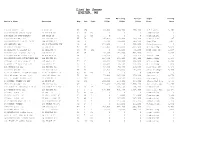
List by Owner EXETER, NH
List by Owner EXETER, NH Land Building Parcel Style Living Owner's Name Location Map Lot Unit Value Value Value Desc Area ______________________________________________________________________________________________________________________________________________________ 1 CASS STREET LLC 1 CASS ST 63 274 91,600 284,700 376,300 4-7 Family 3,163 10 EPPING RD CONDO ASSOC 10 EPPING RD 63 38 MC 0 0 0 Condominium 0 100 HIGH ST CONDOMINIUM 100 HIGH ST 71 51 MC 0 0 0 Condominium 0 103 EPPING ROAD LLC 103 EPPING RD 55 70 105,900 102,400 208,300 Conventional 1,197 107 WATSON RD REALTY TRUST 109 WATSON RD 19 14 137,400 250,800 388,200 Cape Cod 1,977 108 HEIGHTS LLC 108 PORTSMOUTH AVE 52 52 366,200 0 366,200 Petroleum/Gas 0 11 COURT STREET LLC 11 COURT ST 72 158 221,900 840,000 1,061,900 Office Bldg 15,036 11 HEMLOCK ST EXETER NH 11 HEMLOCK ST 95 64 182 0 51,200 51,200 Manf Home DW 1,108 117 WATER ST PROPERTIES LLC 115 WATER ST 72 24 158,000 243,800 401,800 Store 3,504 12 CONTINENTAL DRIVE LLC 60 GOURMET PL 46 1 310,128 0 310,128 Vacant Land 0 120 EPPING ROAD INVESTMENT LLC 120 EPPING RD 55 7 286,500 233,900 520,400 Office Bldg 2,954 127 WATER STREET REALTY LLC 127 WATER ST 72 22 158,000 206,500 364,500 Office Bldg 2,664 129 WATER STREET SPE LLC 129 WATER ST 72 21 97,900 56,500 154,400 Office Bldg 658 131 EPPING RD LLC 131 EPPING RD 55 65 88,800 60,700 149,500 Manf Home DW 1,450 133 EPPING ROAD LLC 133 EPPING RD 55 64 103,800 170,000 273,800 Service Shops 5,465 14 BITTERSWEET LN CONDO ASSOC 14 BITTERSWEET LN 65 67 MC 0 0 0 Condominium 0 141-143 -

EAZA Best Practices Guidelines for Elephants 2020
EAZA Best Practice Guidelines for Elephants second edition published 2020 EAZA Elephant Best Practice Guidelines 2020 Editorial team (in alphabetical order): Petra Bolechova, Zoo Liberec, Czech Republic Marcus Clauss, University of Zurich, Switzerland Danny de Man, EAZA Office Cordula Galeffi, Zürich Zoo, Switzerland Sander Hofman, Antwerpen Zoo, Belgium Jeroen Kappelhof, Rotterdam Zoo, The Netherlands Guy Kfir, Ramat Gan Zoo Bo Kjellson, Boras Zoo, Sweden Thomas Kölpin, Wilhelma Zoo Stuttgart, Germany Arne Lawrenz, Wuppertal Zoo, Germany Imke Lüders, GEOLifes, Germany Andrew McKenzie, Chester Zoo, UK Con Mul, Ouwehands Zoo, The Netherlands Ann-Kathrin Oerke, German Primate Centre Göttingen, Germany Jana Pluhackova, Ostrava Zoo, Czech Republic Fiona Sach, ZSL, UK Willem Schaftenaar, Rotterdam Zoo, The Netherlands Christian Schiffmann, University of Zurich, Switzerland Harald Schmidt, Rotterdam Zoo, The Netherlands Endre Sos, Budapest Zoo, Hungary Lars Versteege, Beekse Bergen, The Netherlands The Editorial team would like to acknowledge that the EAZA Best Practise Guidelines for Elephants (2020) are based on the BIAZA Elephant Management Guidelines (2019), and thus thank the editors and all the contributors of these BIAZA guidelines for the enormous contribution to these EAZA guidelines. Any amendments made to content during development of these EAZA Best Practise Guidelines have not been endorsed by those contributors. EAZA Elephant Taxon Advisory Group core group Chair: Thomas Kölpin, Wilhelma Zoo Stuttgart, Germany Vice-chair: Jana Pluhackova, Ostrava Zoo, Czech Republic Asian elephant EEP coordinator: Harald Schmidt, Rotterdam Zoo, The Netherlands African elephant EEP coordinator: Arne Lawrenz, Wuppertal Zoo, Germany Disclaimer Copyright (2020) by EAZA Executive Office, Amsterdam. All rights reserved. No part of this publication may be reproduced in hard copy, machine-readable or other forms without advance written permission from the European Association of Zoos and Aquaria (EAZA). -

Blunt Force Traumatic Injuries of the Chest
BLUNT FORCE TRAUMATIC INJURIES OF THE CHEST WILLIAM A. COX, M.D. FORENSIC PATHOLOGIST/NEUROPATHOLOGIST January 20, 2012 I. Introduction In this chapter we will review of the anatomy of the thorax and discuss traumatic injuries to the chest wall and the thoracic viscera. Chest wall injuries include the skin, subcutaneous tissue, intercostal muscles, ribs, sternum, and parietal pleura. Thoracic visceral injuries include two main categories: (1) Mechanical injuries of the respiratory system, which will include diaphragmatic rupture. The inclusion of diaphragmatic rupture is due to the fact clinically it presents with symptoms analogous to pneumothorax; (2) Mechanical injuries of the cardiovascular system, which will include the mediastinum. This is primarily due to the fact the most common cause of a widen mediastinum is aortic rupture. We will also discuss the mechanisms of chest injury. II. Musculoskeletal Anatomy of the Thorax A. Overview: The thorax forms the upper part of the trunk, being separated from the lower part, the abdomen, by the diaphragm. The thorax consists of two parts, the musculoskeletal cage (rib and thorax cage) and an internal cavity that contains the lungs, heart, mediastinum, trachea, esophagus, thymus, vagus and phrenic nerves, the right and left sympathetic trunks, the thoracic duct and major systemic and pulmonary blood vessels (see Figs 1, 2 & 3). Fig. 1. Anterior view of human skeleton. (Wiki) 2 Fig. 2. Posterior view of human skeleton. (Wiki) 3 Fig. 3. Surface projections of the organs of the trunk. (Wiki) Posteriorly, the thorax is formed by 12 thoracic vertebrae and the intervertebral disks and the posterior aspect of the ribs (see Fig. -

3/20 Updated Pediatric Protocols (PDF
EMS System Pediatric Pre-hospital Care Manual November 2011 Updated July 2016 – Dr. Neal Rushforth, Medical Director ILS/ALS providers should use the HandTevy System for Pediatric calls. ******If uncertain, revert to weight based protocols.****** Table of Contents Pediatric Assessment Process and Management 1 Pediatric Assessment Triangle (PAT) 2 Pediatric Age Definitions 6 Assessment of the Pediatric Patient 9 Normal Pediatric Vital Sign Ranges 12 Routine Pediatric Care Protocol 13 Pediatric Pain Control Protocol 16 Pediatric Cardiac Arrest Protocol 19 Resuscitation of Pediatric Pulseless Rhythms Protocol 23 V-Fib or Pulseless V-Tach, PEA and Asystole Pediatric Bradycardia Protocol 27 Pediatric Narrow Complex Tachycardia Protocol 29 Pediatric Wide Complex Tachycardia Protocol 31 Pediatric Respiratory Distress Protocol 33 Pediatric Tracheostomy Protocol 36 Pediatric Respiratory Arrest Protocol 38 Pediatric Altered Level of Consciousness Protocol 40 Pediatric Seizure Protocol 42 Pediatric Allergic Reaction / Anaphylaxis Protocol 45 Pediatric Ingestion / Overdose / Toxic Exposure Protocol 48 Routine Pediatric Trauma Care Protocol 50 Pediatric Shock Protocol 56 Pediatric Closed Head Injury Protocol 58 Pediatric Burn Protocol 60 Pediatric Heat-Related Emergencies Protocol 66 Pediatric Acute Nausea and Vomiting 68 Pediatric Hypothermia Protocol 71 Pediatric Drowning Protocol 73 Suspected Child Maltreatment Protocol 75 Sudden Infant Death Syndrome (SIDS) Protocol 76 Pediatric Assessment Process and Management A patient the age of fifteen (15) and under is considered to be a pediatric patient. Utilization of pediatric treatment guidelines and the extent of care rendered is based on the general impression of the pediatric patient’s condition, physical examination findings and the history of the event. Patients 16 years or older will treated with adult protocols. -

List by Owner EXETER, NH
List by Owner EXETER, NH Land Building Parcel Style Living Owner's Name Location Map Lot Unit Value Value Value Desc Area ______________________________________________________________________________________________________________________________________________________ 1 CASS STREET LLC 1 CASS ST 63 274 132,800 304,400 437,200 4-7 Family 3,023 10 ARBOR STREET LLC 10 ARBOR ST 73 32 131,800 147,600 279,400 Three Family 1,503 10 EPPING RD CONDO ASSOC 10 EPPING RD 63 38 MC 0 0 0 Condominium 0 100 DOMAIN DRIVE DD LLC 88.98% 100 DOMAIN DR 88 5 2,256,000 18,110,600 20,366,600 Office Bldg 263,990 100 HIGH ST CONDOMINIUM 100 HIGH ST 71 51 MC 0 0 0 Condominium 0 103 EPPING ROAD LLC 103 EPPING RD 55 70 142,500 0 142,500 Vacant Land 0 107 WATSON ROAD RELATY TR 107 WATSON RD 19 13 189,800 173,600 363,400 Two Family 1,064 108 HEIGHTS LLC 108 PORTSMOUTH AVE 52 52 439,500 178,100 617,600 Car Wash 3,100 109 WATSON ROAD REALTY TR 109 WATSON RD 19 14 176,200 275,100 451,300 Cape Cod 1,977 11 COURT ST UNITS 1 & 2 LLC 11 COURT ST UNIT 1 72 158 1 0 387,900 387,900 Condo Office 3,082 11 COURT ST UNITS 1 & 2 LLC 11 COURT ST UNIT 2 72 158 2 0 387,900 387,900 Condo Office 3,082 117 WATER ST PROPERTIES LLC 115 WATER ST 72 24 232,800 243,800 476,600 Store 3,504 119-121 FRONT STREET LLC 119-121 FRONT ST 73 215 123,600 220,600 344,200 Two Family 2,947 12 CONTINENTAL DRIVE LLC 60 GOURMET PL 46 1 589,000 6,528,900 7,117,900 Light Indust 106,476 12 HAMPTON FALLS RD 12 HAMPTON FALLS RD 86 16 MC 0 0 0 Condominium 0 12 LINCOLN ST LLC 12 LINCOLN ST 73 262 180,200 331,300 -

Chapter and Surgical Procedures (Section 0) 47
Abstracting for Medical Chapter and Surgical Procedures (Section 0) 47 Learning Objectives Chapter Outline After completing this chapter, you should have the skills to: • Medical and Surgical 47.1 Spell and define the key words, medical terms, and abbreviations related to Procedure Basics medical and surgical procedures. (Remember) • Coding Guidelines for 47.2 Adhere to PCS guidelines for Medical and Surgical procedures. (Apply) Medical and Surgical 47.3 Examine and abstract information from the medical record for each character Procedures of Medical and Surgical procedures. (Analyze) • Abstracting Medical and Surgical Procedures Key Terms and Abbreviations diagnostic procedure operative report therapeutic procedure Via Natural or Artificial Opening divided Percutaneous Via Natural or Artificial Opening Endoscopic with Percutaneous External Percutaneous Endoscopic Via Natural or Artificial Opening Endoscopic Assistance Open procedure report Endoscopic In addition to the key terms listed here, students should know the terms defined within tables in this chapter. M47_PAPA8801_02_SE_C47.indd 971 18/10/2018 19:38 972 SECTION FOUR ICD-10-PCS Procedure Coding INTRODUCTION proven effectiveness of one approach over others, and other fac- When you visit a new city, you might first go to the visitor’s tors. In some cases, the surgeon may plan to use one approach information center to gather some general information about then need to change to another approach due to complicat- the area before exploring individual attractions. Your introduc- ing factors. For example, the surgeon may plan to perform an tion to the PCS Medical and Surgical Section is presented in endoscopic cholecystectomy, but due to adhesions must change two chapters. -

War Chatter —From Gathering Noise from My Life: a Camouflaged Memoir
Donald Anderson War Chatter —from Gathering Noise from My Life: A Camouflaged Memoir This essay first appeared in 2011 in a shortened form in “Casualty,” a Special Issue of The Massachusetts Review n our home, there was plenty of aspirin and Vitamin C. Apples and turnips (and canned beets and peas) in the root cellar, a converted bomb shelter. My father had not finished pouring the concrete walls or installing the lead ceiling. IThe ventilation system remained what my father termed aquandary . You may not be interested in war, but war is interested in you. —Leon Trotsky Eddie Turner, Jackie Baker, and I lobbed dirt clods at Mrs. Lorango’s wet sheets every wash day until we got caught. We were surprised that Mrs. Lorango knew we were the throwers and that our parents, without checking with us, believed her. We’d hid behind a berm in the cow pasture where we pretended we were pulling grenade pins with our teeth. My father’s best friend Sidney died at Pearl Harbor. My father had tried to join up but had been denied enlistment because of a childhood mishap in his father’s wood yard that had blinded one eye. My father had wanted to join the Navy before the attack on Pearl Harbor, explaining that if he and Sidney had been able to enlist together, they could have asked to be assigned to the same ship. Whatever would have happened to my father on board Sidney’s ship would have happened more than four years before my birth. Donald Arthur Anderson is interred in Butte. -
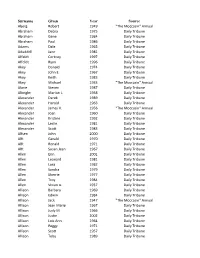
Surname Given Year Source Aberg Robert 1949 "The Moccasin
Surname Given Year Source Aberg Robert 1949 "The Moccasin" Annual Abraham Debra 1975 Daily Tribune Abraham Gene 1984 Daily Tribune Abraham Paul 1986 Daily Tribune Adams Dale 1965 Daily Tribune Aduddell Jane 1981 Daily Tribune Affeldt Cortney 1997 Daily Tribune Affeldt Ryan 1996 Daily Tribune Akey Donald 1974 Daily Tribune Akey John E. 1967 Daily Tribune Akey Keith 1983 Daily Tribune Akey Michael 1933 "The Moccasin" Annual Alarie Steven 1987 Daily Tribune Albright Marion L. 1938 Daily Tribune Alexander Derek 1989 Daily Tribune Alexander Harold 1963 Daily Tribune Alexander James V. 1956 "The Moccasin" Annual Alexander Joan 1960 Daily Tribune Alexander Kristine 1992 Daily Tribune Alexander Leslie 1981 Daily Tribune Alexander Scott 1983 Daily Tribune Alfsen John 2000 Daily Tribune Alft Gerald 1970 Daily Tribune Alft Ronald 1971 Daily Tribune Alft Susan Jean 1967 Daily Tribune Allen Erin 2001 Daily Tribune Allen Leonard 1981 Daily Tribune Allen Lora 1982 Daily Tribune Allen Sandra 1979 Daily Tribune Allen Sherrie 1977 Daily Tribune Allen Troy 1984 Daily Tribune Allen Vivian A. 1937 Daily Tribune Allison Barbara 1969 Daily Tribune Allison Edwin 1984 Daily Tribune Allison Jack 1947 "The Moccasin" Annual Allison Jean Marie 1967 Daily Tribune Allison Judy M. 1966 Daily Tribune Allison Justin 2002 Daily Tribune Allison Lois Ann 1964 Daily Tribune Allison Peggy 1971 Daily Tribune Allison Scott 1957 Daily Tribune Allison Toby 1989 Daily Tribune Alliston Art 1968 Daily Tribune Alloway Kay 1953 "The Moccasin" Annual Alloway Marilyn 1960 Daily Tribune -

An Integrated Approach to Understanding the Role of the Long Neck in Plesiosaurs
An integrated approach to understanding the role of the long neck in plesiosaurs LESLIE F. NOÈ, MICHAEL A. TAYLOR, and MARCELA GÓMEZ-PÉREZ Noè, L.F., Taylor, M.A., and Gómez-Pérez, M. 2017. An integrated approach to understanding the role of the long neck in plesiosaurs. Acta Palaeontologica Polonica 62 (1): 137–162. The evolution and function of the long neck in plesiosaurs, and how the problems associated with stiffness or flexibility were overcome during feeding, or rapid swimming during predator avoidance, are explored, and a new interpretation for the function of the plesiosaur neck is presented. Based on the anatomy of the articular faces of contiguous cervical vertebral centra, neural arches, and cervical ribs, the plesiosaur neck was mainly adapted for ventral bending, with dorsal, lateral and rotational movements all relatively restricted. Predominant ventral bending indicates the neck was adapted for use beneath the body, suggesting feeding in the water column, close to the sea floor, or within soft sediments on the sea floor. A new model is proposed for the plesiosaur bauplan, comprising the head as a filter, straining, sieve feeding or sediment raking apparatus, mounted on a neck which acted as a stiff but ventrally flexible feeding tube, attached to the body which acted as a highly mobile feeding platform. Numerous features of plesiosaurs, including cranial and dental form, cervical vertebral morphology, body shape and limb-based propulsion, conform to this model. Comparative data from modern organisms support this novel explanation for the structure and function of the plesiosaur long neck. This integrative analysis offers an explanation for the evolution of the plesiosaur long neck as a key evolutionary novelty, and why this apparently enigmatic feature remained a prominent feature of plesiosaurs throughout their long evolutionary history.