An Integrated Approach to Understanding the Role of the Long Neck in Plesiosaurs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cranial Anatomy, Taxonomic Implications
[Palaeontology, Vol. 55, Part 4, 2012, pp. 743–773] CRANIAL ANATOMY, TAXONOMIC IMPLICATIONS AND PALAEOPATHOLOGY OF AN UPPER JURASSIC PLIOSAUR (REPTILIA: SAUROPTERYGIA) FROM WESTBURY, WILTSHIRE, UK by JUDYTH SASSOON1, LESLIE F. NOE` 2 and MICHAEL J. BENTON1* 1School of Earth Sciences, University of Bristol, Wills Memorial Building, Queen’s Road, Bristol BS8 1RJ, UK; e-mails: [email protected], [email protected] 2Geociencias, departamento de Fisica, Universidad de los Andes, Bogota´ DC, Colombia; e-mail: [email protected] *Corresponding author. Typescript received 5 December 2010; accepted in revised form 6 April 2011 Abstract: Complete skulls of giant marine reptiles of the genera. The two Westbury Pliosaurus specimens share many Late Jurassic are rare, and so the discovery of the 1.8-m- features, including the form of the teeth, but marked differ- long skull of a pliosaur from the Kimmeridge Clay Forma- ences in the snout and parietal crest suggest sexual dimor- tion (Kimmeridgian) of Westbury, Wiltshire, UK, is an phism; the present specimen is probably female. The large important find. The specimen shows most of the cranial size of the animal, the extent of sutural fusion and the and mandibular anatomy, as well as a series of pathological pathologies suggest this is an ageing individual. An erosive conditions. It was previously referred to Pliosaurus brachy- arthrotic condition of the articular glenoids led to pro- spondylus, but it can be referred reliably only to the genus longed jaw misalignment, generating a suite of associated Pliosaurus, because species within the genus are currently in bone and dental pathologies. -

CSMS General Assembly Thursday, December 20, 7:00 PM Annual
Colorado Springs Mineralogical Society Founded in 1936 Lazard Cahn Honorary President December 2018 PICK&PACK Vol 58 .. Number #10 Inside this Issue: CSMS General Assembly CSMS Calendar Pg. 2 Kansas Pseudo- Morphs: Pg. 4 Thursday, December 20, 7:00 PM Limonite After Pyrite Duria Antiquior: A Annual Christmas Party 19th Century Fore- Pg. 7 runner of Paleoart CSMS Rockhounds of Pg. 9 the Year & 10 Pebble Pups Pg10 **In case of inclement weather, please call** Secretary’s Spot Pg11 Mt. Carmel Veteran’s Service Center 719 309-4714 Classifieds Pg17 Annual Christmas Party Details Please mark your calendars and plan to attend the annual Christmas Party at the Mt. Carmel Veteran's Service Center.on Thursday, December 20, at 7PM. We will provide roast turkey and baked ham as well as Bill Arnson's chili. Members whose last names begin with A--P, please bring a side dish to share. Those whose last names begin with Q -- Z, please bring a dessert to share. There will be a gift exchange for those who want to participate. Please bring a wrapped hobby-related gift valued at $10.00 or less to exchange. We will have a short business meeting to vote on some bylaw changes, and to elect next year's Board of Directors. The Board candidates are as follows: President: Sharon Holte Editor: Taylor Harper Vice President: John Massie Secretary: ?????? Treasurer: Ann Proctor Member-at-Large: Laurann Briding Membership Secretary: Adelaide Barr Member-at Large: Bill Arnson Past president: Ernie Hanlon We are in desperate need of a secretary to serve on the Board. -

First Occurrence of a Gigantic Pliosaurid Plesiosaur in The
Bull. Soc. géol. Fr., 2003, t. 174, no 3, pp. 271-278 First occurrence of a gigantic pliosaurid plesiosaur in the late Jurassic (Kimmeridgian) of Mexico MARIE-CÉLINE BUCHY1,EBERHARD FREY2,WOLFGANG STINNESBECK1 &JOSÉ GUADALUPE LÓPEZ-OLIVA3 Key words. – Kimmeridgian, Pliosauridae, Mexico, Palaeobiogeography. Abstract. – Reinvestigation of a partial vertebral column from the Kimmeridgian La Caja Formation of Mexico, housed in the University of Linares (Mexico), and previously attributed to a dinosaur, proves to be from a very large pliosaurid plesiosaur. This specimen represents the first plesiosaur described from the Jurassic of Mexico. Its length has been esti- mated at 15 metres and, as a juvenile, is considered to be one of the largest Jurassic marine reptiles. The remains of this animal are here described. The morphology of the vertebral column is not diagnostic beyond family level. Large pliosaur vertebrae of a similar size are known from the Upper Jurassic of Europe, and are often referred to the genera Liopleurodon or Simolestes but these identifications are based only upon the size of the centra and have no taxonomic justification. A portion of rostrum with teeth was discovered together with the vertebral column but is unfortunately now lost. The Mexican pliosaur fills geographical and chronological gaps between western Tethys and South American pliosaurs, and is an additional support to the hypothesis of a Hispanic corridor linking at least temporarily the NW Euro- pean marine province with the western South American marine (Pacific) realm during the late Jurassic. Première occurrence d’un plésiosaure pliosauride géant dans le Jurassique supérieur (Kimméridgien) du Mexique Mots clés. -
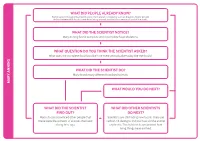
What Did the Scientist Notice? What Question Do You Think
WHAT DID PEOPLE ALREADY KNOW? Some people thought that fossils came from ancient creatures such as dragons. Some people did not believe that fossils came from living animals or plants but were just part of the rocks. WHAT DID THE SCIENTIST NOTICE? Mary Anning found complete and incomplete fossil skeletons. WHAT QUESTION DO YOU THINK THE SCIENTIST ASKED? What does the complete fossil look like? Are there animals alive today like the fossils? WHAT DID THE SCIENTIST DO? Mary found many different fossilised animals. MARY ANNING MARY WHAT WOULD YOU DO NEXT? WHAT DID THE SCIENTIST WHAT DID OTHER SCIENTISTS FIND OUT? DO NEXT? Mary’s fossils convinced other people that Scientists are still finding new fossils. They use these were the remains of animals that lived carbon-14 dating to find out how old the animal a long time ago. or plant is. This helps us to understand how living things have evolved. s 1823 / 1824 1947 Mary Anning (1823) discovered a nearly complete plesiosaur Carbon-14 dating enables scientists to determine the age of a formerly skeleton at Lyme Regis. living thing more accurately. When a Tail fossils of a baby species William Conybeare (1824) living organism dies, it stops taking in of Coelurosaur, fully People often found fossils on the beach described Mary’s plesiosaur to the new carbon. Measuring the amount preserved in amber including and did not know what they were, so Geographical Society. They debated of 14C in a fossil sample provides soft tissue, were found in 2016 BEFORE 1800 BEFORE gave them interesting names such as whether it was a fake, but Mary was information that can be used to Myanmar. -

O'keefe, F. R. 2006
12 Neoteny and the Plesiomorphic Condition of the Plesiosaur Basicranium F. Robin O’Keefe Introduction Historically, the systematics of the Plesiosauria (Reptilia, Sauroptery- gia) were based largely on postcranial characters (Persson, 1963; Brown, 1981). Several factors account for this bias: plesiosaur skulls tend to be del- icate and are often crushed even when preserved, postcranial elements are relatively common and cranial elements are not, lack of knowledge about the relationships of stem-group sauropterygians, and lack of knowledge of plesiosaur cranial anatomy itself. However, recent detailed examinations of plesiosaur cranial anatomy have identified many characters of use in plesiosaur systematics (Brown, 1993; Cruickshank, 1994; Storrs & Taylor, 1996; Storrs, 1997; Carpenter, 1997; Evans, 1999; O’Keefe, 2001, 2004), and the systematics of the group have changed markedly in response (Carpenter, 1997; O’Keefe, 2001, 2004). The work of Rieppel and others has clarified the anatomy and relationships of stem-group sauropterygians (Storrs, 1991; see Rieppel, 2000, for review). This work has laid the anatomic and phylogenetic foundations for a better understanding of ple- siosaur cranial anatomy. The purpose of this paper is to describe the condition of the braincase in stratigraphically early and morphologically primitive plesiosaurs. In- formation on the braincase of plesiomorphic taxa is important because it establishes the polarity of characters occurring in more derived ple- siosaurs. This paper begins with a short review of braincase anatomy in stem-group sauropterygians. Data on braincase morphology of the ple- siomorphic plesiosaur genera Thalassiodracon and Eurycleidus are then presented and interpreted via comparison with other plesiosaurs, stem- group sauropterygians, and stem diapsids (Araeoscelis). -

Mars, Mammon--And Other Options Carl Skrade
Intersections Volume 2004 | Number 20 Article 4 2004 Mars, Mammon--and Other Options Carl Skrade Follow this and additional works at: http://digitalcommons.augustana.edu/intersections Augustana Digital Commons Citation Skrade, Carl (2004) "Mars, Mammon--and Other Options," Intersections: Vol. 2004: No. 20, Article 4. Available at: http://digitalcommons.augustana.edu/intersections/vol2004/iss20/4 This Article is brought to you for free and open access by Augustana Digital Commons. It has been accepted for inclusion in Intersections by an authorized administrator of Augustana Digital Commons. For more information, please contact [email protected]. Mars, Mammon---and Other Options Carl Skrade Woe to those who go down to Egypt forhelp And rely on horses, Who trust in chariots because they are many And in horsemen because they are very strong, But do not look to the holy one of Israel Or consult the Lord! -Isaiah 31: 1 Blessed are the peacemakers, forthey shall be called the sons of God. -Matthew 5: 9 Overgrown military establishments are under any formof government inauspicious to liberty, and are to be regarded as particularly hostile to Republican liberty. -George Washington, Farewell Address, September 17, 1796 The conjunctionof an immense military establishment and a large arms industry is new in the American experience .... In the councils of government, we must guard against the acquisition of unwarranted influence, whether sought or unsought, by the military-industrial complex. The potential for disastrous rise of misplaced power exists and will persist. We must never let the weight of this combination endanger our liberties or democratic processes. We should take nothing forgranted. -

Cryptoclidid Plesiosaurs (Sauropterygia, Plesiosauria) from the Upper Jurassic of the Atacama Desert
Journal of Vertebrate Paleontology ISSN: (Print) (Online) Journal homepage: https://www.tandfonline.com/loi/ujvp20 Cryptoclidid plesiosaurs (Sauropterygia, Plesiosauria) from the Upper Jurassic of the Atacama Desert Rodrigo A. Otero , Jhonatan Alarcón-Muñoz , Sergio Soto-Acuña , Jennyfer Rojas , Osvaldo Rojas & Héctor Ortíz To cite this article: Rodrigo A. Otero , Jhonatan Alarcón-Muñoz , Sergio Soto-Acuña , Jennyfer Rojas , Osvaldo Rojas & Héctor Ortíz (2020): Cryptoclidid plesiosaurs (Sauropterygia, Plesiosauria) from the Upper Jurassic of the Atacama Desert, Journal of Vertebrate Paleontology, DOI: 10.1080/02724634.2020.1764573 To link to this article: https://doi.org/10.1080/02724634.2020.1764573 View supplementary material Published online: 17 Jul 2020. Submit your article to this journal Article views: 153 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=ujvp20 Journal of Vertebrate Paleontology e1764573 (14 pages) © by the Society of Vertebrate Paleontology DOI: 10.1080/02724634.2020.1764573 ARTICLE CRYPTOCLIDID PLESIOSAURS (SAUROPTERYGIA, PLESIOSAURIA) FROM THE UPPER JURASSIC OF THE ATACAMA DESERT RODRIGO A. OTERO,*,1,2,3 JHONATAN ALARCÓN-MUÑOZ,1 SERGIO SOTO-ACUÑA,1 JENNYFER ROJAS,3 OSVALDO ROJAS,3 and HÉCTOR ORTÍZ4 1Red Paleontológica Universidad de Chile, Laboratorio de Ontogenia y Filogenia, Departamento de Biología, Facultad de Ciencias, Universidad de Chile, Las Palmeras 3425, Santiago, Chile, [email protected]; 2Consultora Paleosuchus Ltda., Huelén 165, Oficina C, Providencia, Santiago, Chile; 3Museo de Historia Natural y Cultural del Desierto de Atacama. Interior Parque El Loa s/n, Calama, Región de Antofagasta, Chile; 4Facultad de Ciencias Naturales y Oceanográficas, Universidad de Concepción, Barrio Universitario, Concepción, Región del Bío Bío, Chile ABSTRACT—This study presents the first plesiosaurs recovered from the Jurassic of the Atacama Desert that are informative at the genus level. -

Estimating the Evolutionary Rates in Mosasauroids and Plesiosaurs: Discussion of Niche Occupation in Late Cretaceous Seas
Estimating the evolutionary rates in mosasauroids and plesiosaurs: discussion of niche occupation in Late Cretaceous seas Daniel Madzia1 and Andrea Cau2 1 Department of Evolutionary Paleobiology, Institute of Paleobiology, Polish Academy of Sciences, Warsaw, Poland 2 Independent, Parma, Italy ABSTRACT Observations of temporal overlap of niche occupation among Late Cretaceous marine amniotes suggest that the rise and diversification of mosasauroid squamates might have been influenced by competition with or disappearance of some plesiosaur taxa. We discuss that hypothesis through comparisons of the rates of morphological evolution of mosasauroids throughout their evolutionary history with those inferred for contemporary plesiosaur clades. We used expanded versions of two species- level phylogenetic datasets of both these groups, updated them with stratigraphic information, and analyzed using the Bayesian inference to estimate the rates of divergence for each clade. The oscillations in evolutionary rates of the mosasauroid and plesiosaur lineages that overlapped in time and space were then used as a baseline for discussion and comparisons of traits that can affect the shape of the niche structures of aquatic amniotes, such as tooth morphologies, body size, swimming abilities, metabolism, and reproduction. Only two groups of plesiosaurs are considered to be possible niche competitors of mosasauroids: the brachauchenine pliosaurids and the polycotylid leptocleidians. However, direct evidence for interactions between mosasauroids and plesiosaurs is scarce and limited only to large mosasauroids as the Submitted 31 July 2019 predators/scavengers and polycotylids as their prey. The first mosasauroids differed Accepted 18 March 2020 from contemporary plesiosaurs in certain aspects of all discussed traits and no evidence Published 13 April 2020 suggests that early representatives of Mosasauroidea diversified after competitions with Corresponding author plesiosaurs. -

Mary Anning (1799 – 1847) Was One of the First Fossil Collectors
Geology Section: history, interests and the importance of Devon‟s geology Malcolm Hart [Vice-Chair Geology Section] School of Geography, Earth & Environmental Sciences, University of Plymouth Devonshire Association, Forum, Sidmouth, March 2020 Slides: 3 – 13 South-West England people; 14 – 25 Our northward migration; 24 – 33 Climate change: a modern problem; 34 – 50 Our geoscience heritage: Jurassic Coast World Heritage Site and the English Riviera UNESCO Global Geopark; 51 – 53 Summary and perspectives When we look at the natural landscape it can appear almost un- changing – even in the course of a life- time. William Smith (1769–1839) was a practical engineer, who used geology in an applied way. He recognised that the fossils he found could indicate the „stratigraphy‟ of the rocks that his work encountered. His map was produced in 1815. Images © Geological Society of London Mary Anning (1799 – 1847) was one of the first fossil collectors. At the time the area was being quarried, though features such as the „ammonite pavement‟ were left for science. Image © Geological Society of London Images © National Museum, Wales Sir Henry De La Beche (1796‒1855) was an extraordinary individual. He wrote on the geology of Devon, Cornwall and Somerset, while living in Lyme Regis. He studied the local geology and created, in Duria Antiquior (a more ancient Dorset) the first palaeoecological reconstruction. He was often ridiculed for this! He was also the first Director of the British Geological Survey. His suggestion, in 1839, that the rocks of Devonshire and Cornwall were „distinctive‟ led to the creation of the Devonian System in 1840. -

An Mauisaurus Gardneri On
Downloaded from http://jgslegacy.lyellcollection.org/ at University of Sydney on August 31, 2014 Quarterly Journal of the Geological Society On Mauisaurus Gardneri (Seeley), an Elasmosaurian from the Base of the Gault at Folkestone Harry Govier Seeley Quarterly Journal of the Geological Society 1877, v.33; p541-547. doi: 10.1144/GSL.JGS.1877.033.01-04.32 Email alerting click here to receive free service e-mail alerts when new articles cite this article Permission click here to seek permission request to re-use all or part of this article Subscribe click here to subscribe to Quarterly Journal of the Geological Society or the Lyell Collection Notes © The Geological Society of London 2014 Downloaded from http://jgslegacy.lyellcollection.org/ at University of Sydney on August 31, 2014 H. G0VIER SEELEY ON MAUISAURUS GARDNERL 541 29. On ]~AUISAURUS GARDN]~RI (Seele~), a~ ELAS]IiOSAURIANfro~}~ the Bxs~ of the GAcT.~ aS FOT.K~S~O~. By HARa~ GOVmR SEX,Y, :Esq., F.L.S., F.G.S., &e., Professor of Geography in King's Col- lege, London. (Read February 7, 1877.) [PLATE XXIII.] Tnm Gault hitherto has yie]ded but scanty remains of animals re- ferable to the Reptilia and to the Pal~eosauria; so that more than ordinary interest attaches to the discovery, in a comparatively per- fect condition, of remains belonging to a genus found hitherto only in New Zealand, which may be regarded as distinctive of the de- posit. The remains of this Plesiosaurian were first found, rolled and abraded, at the foot of the cliffs; much of the caudal region of the animal may therefore have disappeared by attrition, and by the gradual decay of the bones as exposed in the clay, which has partly invested them with selenite. -

Description of an Unusual Cervical Vertebral Column of a Plesiosaur from the Kiowa Shale Ian N
Fort Hays State University FHSU Scholars Repository Master's Theses Graduate School Spring 2014 Description of an Unusual Cervical Vertebral Column of a Plesiosaur from the Kiowa Shale Ian N. Cost Fort Hays State University Follow this and additional works at: https://scholars.fhsu.edu/theses Part of the Biology Commons Recommended Citation Cost, Ian N., "Description of an Unusual Cervical Vertebral Column of a Plesiosaur from the Kiowa Shale" (2014). Master's Theses. 57. https://scholars.fhsu.edu/theses/57 This Thesis is brought to you for free and open access by the Graduate School at FHSU Scholars Repository. It has been accepted for inclusion in Master's Theses by an authorized administrator of FHSU Scholars Repository. DESCRIPTION OF AN UNUSUAL CERVICAL VERTEBRAL COLUMN OF A PLESIOSAUR FROM THE KIOWA SHALE being A Thesis Presented to the Graduate Faculty of the Fort Hays State University in Partial Fulfillment of the Requirements for the Degree of Master of Science by Ian Cost B.A., Bridgewater State University M.Ed., Lesley University Date_____________________ Approved________________________________ Major Professor Approved________________________________ Chair, Graduate Council This Thesis for The Master of Science Degree By Ian Cost Has Been Approved __________________________________ Chair, Supervisory Committee __________________________________ Supervisory Committee __________________________________ Supervisory Committee __________________________________ Supervisory Committee __________________________________ Supervisory Committee __________________________________ Chair, Department of Biological Science i PREFACE This manuscript has been formatted in the style of the Journal of Vertebrate Paleontology. Keywords: plesiosaur, polycotylid, cervical vertebrae, Dolichorhynchops, Trinacromerum ii ABSTRACT The Early Cretaceous (Albian) Kiowa Shale of Clark County, Kansas consists mainly of dark gray shale with occasional limestone deposits that represent a near shore environment. -
Reptile Family Tree
Reptile Family Tree - Peters 2015 Distribution of Scales, Scutes, Hair and Feathers Fish scales 100 Ichthyostega Eldeceeon 1990.7.1 Pederpes 91 Eldeceeon holotype Gephyrostegus watsoni Eryops 67 Solenodonsaurus 87 Proterogyrinus 85 100 Chroniosaurus Eoherpeton 94 72 Chroniosaurus PIN3585/124 98 Seymouria Chroniosuchus Kotlassia 58 94 Westlothiana Casineria Utegenia 84 Brouffia 95 78 Amphibamus 71 93 77 Coelostegus Cacops Paleothyris Adelospondylus 91 78 82 99 Hylonomus 100 Brachydectes Protorothyris MCZ1532 Eocaecilia 95 91 Protorothyris CM 8617 77 95 Doleserpeton 98 Gerobatrachus Protorothyris MCZ 2149 Rana 86 52 Microbrachis 92 Elliotsmithia Pantylus 93 Apsisaurus 83 92 Anthracodromeus 84 85 Aerosaurus 95 85 Utaherpeton 82 Varanodon 95 Tuditanus 91 98 61 90 Eoserpeton Varanops Diplocaulus Varanosaurus FMNH PR 1760 88 100 Sauropleura Varanosaurus BSPHM 1901 XV20 78 Ptyonius 98 89 Archaeothyris Scincosaurus 77 84 Ophiacodon 95 Micraroter 79 98 Batropetes Rhynchonkos Cutleria 59 Nikkasaurus 95 54 Biarmosuchus Silvanerpeton 72 Titanophoneus Gephyrostegeus bohemicus 96 Procynosuchus 68 100 Megazostrodon Mammal 88 Homo sapiens 100 66 Stenocybus hair 91 94 IVPP V18117 69 Galechirus 69 97 62 Suminia Niaftasuchus 65 Microurania 98 Urumqia 91 Bruktererpeton 65 IVPP V 18120 85 Venjukovia 98 100 Thuringothyris MNG 7729 Thuringothyris MNG 10183 100 Eodicynodon Dicynodon 91 Cephalerpeton 54 Reiszorhinus Haptodus 62 Concordia KUVP 8702a 95 59 Ianthasaurus 87 87 Concordia KUVP 96/95 85 Edaphosaurus Romeria primus 87 Glaucosaurus Romeria texana Secodontosaurus