Appendicular Skeletal Morphology in Minute Salamanders
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca
Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN or other participating organizations. Published by: IUCN, Gland, Switzerland Copyright: © 2015 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Lamoreux, J. F., McKnight, M. W., and R. Cabrera Hernandez (2015). Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca. Gland, Switzerland: IUCN. xxiv + 320pp. ISBN: 978-2-8317-1717-3 DOI: 10.2305/IUCN.CH.2015.SSC-OP.53.en Cover photographs: Totontepec landscape; new Plectrohyla species, Ixalotriton niger, Concepción Pápalo, Thorius minutissimus, Craugastor pozo (panels, left to right) Back cover photograph: Collecting in Chamula, Chiapas Photo credits: The cover photographs were taken by the authors under grant agreements with the two main project funders: NGS and CEPF. -
Comparative Osteology and Evolution of the Lungless Salamanders, Family Plethodontidae David B
COMPARATIVE OSTEOLOGY AND EVOLUTION OF THE LUNGLESS SALAMANDERS, FAMILY PLETHODONTIDAE DAVID B. WAKE1 ABSTRACT: Lungless salamanders of the family Plethodontidae comprise the largest and most diverse group of tailed amphibians. An evolutionary morphological approach has been employed to elucidate evolutionary rela tionships, patterns and trends within the family. Comparative osteology has been emphasized and skeletons of all twenty-three genera and three-fourths of the one hundred eighty-three species have been studied. A detailed osteological analysis includes consideration of the evolution of each element as well as the functional unit of which it is a part. Functional and developmental aspects are stressed. A new classification is suggested, based on osteological and other char acters. The subfamily Desmognathinae includes the genera Desmognathus, Leurognathus, and Phaeognathus. Members of the subfamily Plethodontinae are placed in three tribes. The tribe Hemidactyliini includes the genera Gyri nophilus, Pseudotriton, Stereochilus, Eurycea, Typhlomolge, and Hemidac tylium. The genera Plethodon, Aneides, and Ensatina comprise the tribe Pleth odontini. The highly diversified tribe Bolitoglossini includes three super genera. The supergenera Hydromantes and Batrachoseps include the nominal genera only. The supergenus Bolitoglossa includes Bolitoglossa, Oedipina, Pseudoeurycea, Chiropterotriton, Parvimolge, Lineatriton, and Thorius. Manculus is considered to be congeneric with Eurycea, and Magnadig ita is congeneric with Bolitoglossa. Two species are assigned to Typhlomolge, which is recognized as a genus distinct from Eurycea. No. new information is available concerning Haptoglossa. Recognition of a family Desmognathidae is rejected. All genera are defined and suprageneric groupings are defined and char acterized. Range maps are presented for all genera. Relationships of all genera are discussed. -

Rediscovery at the Type Locality and Detection of a New Population 1José L
Offcial journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(2) [General Section]: 126–132 (e193). Thorius narismagnus (Amphibia: Plethodontidae): rediscovery at the type locality and detection of a new population 1José L. Aguilar-López, 2,*Paulina García-Bañuelos, 3Eduardo Pineda, and 4Sean M. Rovito 1,2,3Red de Biología y Conservación de Vertebrados, Instituto de Ecología, A.C., Carretera antigua a Coatepec 351, El Haya, Xalapa, Veracruz, MEXICO 4Unidad de Genómica Avanzada (Langebio), Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, km 9.6 Libramiento Norte Carretera Irapuato-León, Irapuato, Guanajuato CP 36824, MEXICO Abstract.—Of the 42 Critically Endangered species of plethodontid salamanders that occur in Mexico, thirteen have not been reported in more than ten years. Given the lack of reports since 1976, the minute plethodontid salamander Thorius narismagnus is widely considered as missing. However, this report describes the rediscovery of this minute salamander at the type locality (Volcán San Martín), as well as a new locality on Volcán Santa Marta, 28 km southeast of its previously known distribution, both in the Los Tuxtlas region of Veracruz, Mexico. The localities where T. narismagnus has been found are mature forests in a community reserve on Volcán San Martín and a private reserve on Volcán Santa Marta. The presence of maxillary teeth, generally absent in Thorius, are reported here in some T. narismagnus females. Two efforts which may contribute to the conservation of Thorius narismagnus are the preservation of the cloud forests where this species persists, as well as the determination of the presence and possible effect of chytrid fungus in these populations. -
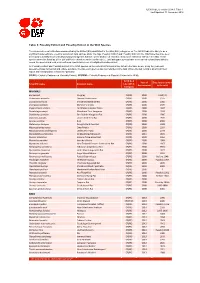
Table 9: Possibly Extinct and Possibly Extinct in the Wild Species
IUCN Red List version 2014.3: Table 9 Last Updated: 13 November 2014 Table 9: Possibly Extinct and Possibly Extinct in the Wild Species The number of recent extinctions documented by the Extinct (EX) and Extinct in the Wild (EW) categories on The IUCN Red List is likely to be a significant underestimate, even for well-known taxa such as birds. The tags 'Possibly Extinct' and 'Possibly Extinct in the Wild' have therefore been developed to identify those Critically Endangered species that are, on the balance of evidence, likely to be extinct (or extinct in the wild). These species cannot be listed as EX or EW until their extinction can be confirmed (i.e., until adequate surveys have been carried out and have failed to record the species and local or unconfirmed reports have been investigated and discounted). All 'Possibly Extinct' and 'Possibly Extinct in the Wild' species on the current IUCN Red List are listed in the table below, along the year each assessment was carried out and, where available, the date each species was last recorded in the wild. Where the last record is an unconfirmed report, last recorded date is noted as "possibly". CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild), IUCN Red Year of Date last recorded Scientific name Common name List (2014) Assessment in the wild Category MAMMALS Bos sauveli Kouprey CR(PE) 2008 1969/70 Crateromys australis Dinagat Crateromys CR(PE) 2008 1975 Crocidura trichura Christmas Island Shrew CR(PE) 2008 1985 Crocidura wimmeri -

Results of the Global Amphibian Assessment for Bolivia
Results of the Global Amphibian Assessment for Bolivia Diversity Number of Rank in Latin World Rank2 Percentage of Species in Bolivia America and the the World’s Caribbean1 Diversity All Amphibians 201 7 15 3.5 % Frogs & Toads 197 7 14 3.9 % Salamanders 1 13 68 0.2 % Caecilians 3 11 20 1.8 % 1 Out of 44 countries and territories. 2 Out of 192 countries and territories. Threatened Species (Threatened species are in one of the categories in italics.) IUCN Categories Number of Species in IUCN Categories Number of Species in Bolivia Bolivia Extinct 0 Near Threatened 6 Critically Endangered 5 Least Concern 161 Endangered 6 Data Deficient 13 Vulnerable 10 Number and percent of Bolivian species that are threatened: 21 (10%) Extinct Species: none Species that are Critically Endangered and possibly extinct (species that are missing but for which sufficient information is lacking to declare them as extinct): Frogs: Gastrotheca lauzuricae, Hyla chlorostea, Eleutherodactylus zongoensis Role of Protected Areas: Of the 21 threatened species in Bolivia, 76% occur in at least one protected area. Local Experts: Steffen Reichle, The Nature Conservancy, TEL (591-3) 348 0766, 348 0767 Claudia Cortez, Colección Boliviana de Fauna, TEL (591-3) 272 1152 For More Information: Dr. Bruce Young, NatureServe, Costa Rica, TEL (506) 645-6231 Global Amphibian Assessment website: www.globalamphibians.org A detailed analysis of the results for the Americas: www.natureserve.org Results of the Global Amphibian Assessment for Bolivia Map of Amphibian Diversity Source: Global Amphibian Assessment Map of the Distribution of Threatened Species Source: Global Amphibian Assessment Results of the Global Amphibian Assessment for Brazil Diversity Number of Rank in Latin World Rank2 Percentage of Species in Brazil America and the the World’s Caribbean1 Diversity All Amphibians 731 1 1 12.7 % Frogs & Toads 704 1 1 13.9 % Salamanders 1 13 68 0.2 % Caecilians 26 2 2 15.5 % 1 Out of 44 countries and territories. -

I Online Supplementary Data – Sexual Size Dimorphism in Salamanders
Online Supplementary data – Sexual size dimorphism in salamanders Supplementary data S1. Species data used in this study and references list. Males Females SSD Significant test Ref Species n SVL±SD n SVL±SD Andrias davidianus 2 532.5 8 383.0 -0.280 12 Cryptobranchus alleganiensis 53 277.4±5.2 52 300.9±3.4 0.084 Yes 61 Batrachuperus karlschmidti 10 80.0 10 84.8 0.060 26 Batrachuperus londongensis 20 98.6 10 96.7 -0.019 12 Batrachuperus pinchonii 5 69.6 5 74.6 0.070 26 Batrachuperus taibaiensis 11 92.9±12.1 9 102.1±7.1 0.099 Yes 27 Batrachuperus tibetanus 10 94.5 10 92.8 -0.017 12 Batrachuperus yenyuadensis 10 82.8 10 74.8 -0.096 26 Hynobius abei 24 57.8±2.1 34 55.0±1.2 -0.048 Yes 92 Hynobius amakusaensis 22 75.4±4.8 12 76.5±3.6 0.014 No 93 Hynobius arisanensis 72 54.3±4.8 40 55.2±4.8 0.016 No 94 Hynobius boulengeri 37 83.0±5.4 15 91.5±3.8 0.102 Yes 95 Hynobius formosanus 15 53.0±4.4 8 52.4±3.9 -0.011 No 94 Hynobius fuca 4 50.9±2.8 3 52.8±2.0 0.037 No 94 Hynobius glacialis 12 63.1±4.7 11 58.9±5.2 -0.066 No 94 Hynobius hidamontanus 39 47.7±1.0 15 51.3±1.2 0.075 Yes 96 Hynobius katoi 12 58.4±3.3 10 62.7±1.6 0.073 Yes 97 Hynobius kimurae 20 63.0±1.5 15 72.7±2.0 0.153 Yes 98 Hynobius leechii 70 61.6±4.5 18 66.5±5.9 0.079 Yes 99 Hynobius lichenatus 37 58.5±1.9 2 53.8 -0.080 100 Hynobius maoershanensis 4 86.1 2 80.1 -0.069 101 Hynobius naevius 72.1 76.7 0.063 102 Hynobius nebulosus 14 48.3±2.9 12 50.4±2.1 0.043 Yes 96 Hynobius osumiensis 9 68.4±3.1 15 70.2±3.0 0.026 No 103 Hynobius quelpaertensis 41 52.5±3.8 4 61.3±4.1 0.167 Yes 104 Hynobius -

Hanken and Wake 1998 Copeia.Pdf
Copeia, 1998(2), pp. 312-345 Biology of Tiny Animals: Systematics of the Minute Salamanders (Thorius: Plethodontidae) from Veracruz and Puebla, Mexico, with Descriptions of Five New Species JAMES HANKEN AND DAVID B. WAKE Minute plethodontid salamanders, genus Thorius, are far more diverse taxonom- ically than has been recognized previously. Populations of these salamanders from the Mexican states of Veracruz and Puebla are assigned to 10 species, five of which are described as new. Combinations of morphological and allozymic characters are used to sort the species and to make initial assessments of relationships. Valid ex- isting names include Thorius pennatulus, T. troglodytes, T. dubitus, and T. schmidti. Thorius narismagnus, from the Sierra de Los Tuxtlas, which previously was consid- ered to be a disjunct subspecies of T. pennatulus, is elevated to species rank. Thorius maxillabrochusis treated as a subjective junior synonym of the sympatric T. schmidti. New taxa include Thorius lunaris, T. magnipes, T. minydemus, T. munificus, and T. spilogaster. All 10 species can be distinguished by morphological characters, but the distinctiveness of the taxa is bolstered by allozymic characters and by extensive sympatry. As many as three, and possibly four, species occur in sympatry, with some evidence of segregation by microhabitat (arboreal vs terrestrial). Adult body sizes span the range known for the genus, from very small in T. pennatulus (maturing at < 16 mm standard length) to large in T. lunaris (adults reaching > 31 mm). Collec- tively these species display a wide elevational distribution, from less than 1000 m (T. pennatulus, T. narismagnus) to more than 3000 m (T lunaris, T. -

Proceedings of the United States National Museum
PROCEEDINGS OF THE UNITED STATES NATIONAL MUSEUM issued i^?fv vl vJ^^S ^V '^^ SMITHSONIAN INSTITUTION U. S. NATIONAL MUSEUM Vol. 95 Washington : 1945 No. 3185 SUMMARY OF THE COLLECTIONS OF AMPHIBIANS MADE IN MEXICO UNDER THE WALTER RATHBONE BACON TRAVELING SCHOLARSHIP By Edward H. Taylor and Hobart M. Smith INTRODUCTION By tenure of the Walter Rathbone Bacon Traveling Scholarship from 1938 to 1940, the junior author was enabled to continue field studies that had been under way several years on the herpetofauna of Mexico. Aided by his wife, he accumulated a collection of reptiles and amphibians the study of which still continues. A brief summary of the snakes and crocodiles has appeared previously (Smith, 1943). With the aid of the senior author a summary of the amphibians has been completed and forms the basis of the present paper. The liz- ards are being studied as time permits, and a summary of them is contemplated. No survey of the turtles is envisioned. The itin- erary and list of localities visited by the collectors will accompany a later report. The amphibians comprise 10,370 specimens, or about half the total number of herpetological specimens obtained. They represent 27 genera and 146 forms. Thirty-three of the species were undescribed at the time of collecting; the specimens of them secured have formed the basis at least in part for their subsequent descriptions. Eleven of the 33 are represented only by paratypes, while 22 are represented by holotypes. Of the latter, eight are described in the present paper, while all others were described by Taylor (1940c, 1941b, d, e, 1942a-d, 1943a, b) or Smith (1939). -

A Statistical Assessment of Population Trends for Data Deficient Mexican Amphibians
A peer-reviewed version of this preprint was published in PeerJ on 16 December 2014. View the peer-reviewed version (peerj.com/articles/703), which is the preferred citable publication unless you specifically need to cite this preprint. Quintero E, Thessen AE, Arias-Caballero P, Ayala-Orozco B. 2014. A statistical assessment of population trends for data deficient Mexican amphibians. PeerJ 2:e703 https://doi.org/10.7717/peerj.703 A statistical assessment of population trends for data deficient Mexican amphibians Esther Quintero, Anne E. Thessen, Paulina Arias-Caballero, Bárbara Ayala-Orozco Background: Mexico is the fourth richest country in amphibians and the second country with the highest quantity of threatened amphibian species, and this number could be higher as many species are too poorly known to be accurately assigned to a risk category. The absence of a risk status or an unknown population trend can slow or halt conservation action, so it is vital to develop tools that in the absence of specific demographic data can assess a species’ risk of extinction, population trend, and to better understand which variables increase their vulnerability. Recent studies have demonstrated that the risk of species decline depends on extrinsic and intrinsic trait, thus including both of them for PrePrints assessing extinction might render more accurate assessment of threat. Methods: In this study harvested data from the Encyclopedia of Life (EOL) and the published literature for Mexican amphibians and used these data to assess the population trend of some of the Mexican species that have been assigned to the Data Deficient category of the IUCN using Random Forests, a Machine Learning method that gives a prediction of complex processes and identifies the most important variables that account for the predictions. -

Feeding in Amphibians: Evolutionary Transformations and Phenotypic Diversity As Drivers of Feeding System Diversity
Chapter 12 Feeding in Amphibians: Evolutionary Transformations and Phenotypic Diversity as Drivers of Feeding System Diversity Anthony Herrel, James C. O’Reilly, Anne-Claire Fabre, Carla Bardua, Aurélien Lowie, Renaud Boistel and Stanislav N. Gorb Abstract Amphibians are different from most other tetrapods because they have a biphasic life cycle, with larval forms showing a dramatically different cranial anatomy and feeding strategy compared to adults. Amphibians with their exceptional diversity in habitats, lifestyles and reproductive modes are also excellent models for studying the evolutionary divergence in feeding systems. In the present chapter, we review the literature on amphibian feeding anatomy and function published since 2000. We also present some novel unpublished data on caecilian feeding biome- chanics. This review shows that over the past two decades important new insights in our understanding of amphibian feeding anatomy and function have been made possible, thanks to a better understanding of the phylogenetic relationships between taxa, analyses of development and the use of biomechanical modelling. In terms of functional analyses, important advances involve the temperature-dependent nature of tongue projection mechanisms and the plasticity exhibited by animals when switch- A. Herrel (B) Département Adaptations du Vivant, Muséum national d’Histoire naturelle, UMR 7179 C.N.R.S/M.N.H.N, 55 rue Buffon, 75005, Paris Cedex 05, France e-mail: [email protected] J. C. O’Reilly Department of Biomedical Sciences, Ohio University, Cleveland Campus, Cleveland, Ohio 334C, USA A.-C. Fabre · C. Bardua Life Sciences Department, The Natural History Museum, Cromwell Road, London SW7 5BD, UK A. Lowie Department of Biology Evolutionary, Morphology of Vertebrates, Ghent University, K.L. -

From the Sierra De Juarez, Oaxaca, Mexico
Herpetologica, 57(4), 2001, 515-523 (? 2001 by The Herpetologists' League, Inc. A SEVENTH SPECIES OF MINUTE SALAMANDER (THORIUS: PLETHODONTIDAE) FROM THE SIERRA DE JUAREZ, OAXACA, MEXICO JAMES HANKEN' AND DAVID B. WAKE2 'Department of Organismic and Evolutionary Biology and Museum of Comparative Zoology, Harvard University, Cambridge, MA 02138, USA 2Department of Integrative Biology and Museumiiof Vertebrate Zoology, University of California, Berkeley, CA 94720-3160, USA ABSTRACT: We describe a new terrestrial species of minute lungless salamander of the Mexican genus Thorius (Plethodontidae) from montane pine-oak forests in the Sierra de Juarez of north central Oaxaca, Mexico. The new species is distinguished from congeners by a combination of body size, external morphology, osteology, and dental traits, and it is well differentiated genetically from other named species for which data are available. This is the seventh endemic species of Thornis reported from the Sierra de Juarez, and known localities are geographically isolated from those of all other species. Discovery of another new species of plethodontid salamander from Oaxaca en- hances the state's standing as a preeminent center of herpetological diversity within both M6xico and Mesoamerica. Key words: Miniaturization; Taxonomy; Biogeography; Osteology MESOAMERICAiS one of the world'sbio- comprised only nine formally described diversity hotspots; both the relative and species (Duellman, 1993). In the last sev- absolute numbers of species and the de- eral years, the number of valid, named gree of endemism are exceeded by few species has more than doubled to 22, in- other terrestrial habitats (Myers et al., cluding five new species described from 2000). Approximately 40% of the nearly Oaxaca (Hanken and Wake, 1994, 1998; 3000 species of tetrapod vertebrates re- Hanken et al., 1999). -

Volume 2, Chapter 14-8: Salamander Mossy Habitats
Glime, J. M. and Boelema, W. J. 2017. Salamander Mossy Habitats. Chapt. 14-8. In: Glime, J. M. Bryophyte Ecology. Volume 2. 14-8-1 Bryological Interaction.Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 19 July 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology2/>. CHAPTER 14-8 SALAMANDER MOSSY HABITATS Janice M. Glime and William J. Boelema TABLE OF CONTENTS Tropical Mossy Habitats – Plethodontidae........................................................................................................ 14-8-3 Terrestrial and Arboreal Adaptations ......................................................................................................... 14-8-3 Bolitoglossa (Tropical Climbing Salamanders) ......................................................................................... 14-8-4 Bolitoglossa diaphora ................................................................................................................................ 14-8-5 Bolitoglossa diminuta (Quebrada Valverde Salamander) .......................................................................... 14-8-5 Bolitoglossa hartwegi (Hartweg's Mushroomtongue Salamander) ............................................................ 14-8-5 Bolitoglossa helmrichi ............................................................................................................................... 14-8-5 Bolitoglossa jugivagans ............................................................................................................................