Chloroformic Acid Ethyl Ester
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hazardous Material Inventory Statement
City of Brooklyn Park FIRE DEPARTMENT 5200 - 85th Avenue North Brooklyn Park MN 55443 Phone: (763)493-8020 Fax: (763) 493-8391 Hazardous Materials Inventory Statement Users Guide A separate inventory statement shall be provided for each building. An amended inventory statement shall be provided within 30 days of the storage of any hazardous materials or plastics that changes or adds a hazard class or which is sufficient in quantity to cause an increase in the quantity which exceeds 5 percent for any hazard class. The hazardous materials inventory statement shall list by hazard class categories. Each grouping shall provide the following information for each hazardous material listed for that group including a total quantity for each group of hazard class. 1. Hazard class. (See attached Hazardous Materials Categories Listing) 2. Common or trade name. 3. Chemical Abstract Service Number (CAS number) found in 29 Code of Federal Regulations (C.F.R.). 4. Whether the material is pure or a mixture, and whether the material is a solid, liquid or gas 5. Maximum aggregate quantity stored at any one time. 6. Maximum aggregate quantity In-Use (Open to atmosphere) at any one time. 7. Maximum aggregate quantity In-Use (Closed to atmosphere) at any one time. 8. Storage conditions related to the storage type, high-pile, encapsulated, non-encapsulated. Attached is a listing of categories that all materials need to be organized to. Definitions of these categories are also attached for your use. At the end of this packet are blank forms for completing this project. For questions regarding Hazardous Materials Inventory Statement contact the Fire Department at 763-493-8020. -

Methyl Chlorocarbonate; CASRN 79-22-1
Integrated Risk Information System (IRIS) U.S. Environmental Protection Agency Chemical Assessment Summary National Center for Environmental Assessment Methyl chlorocarbonate; CASRN 79-22-1 Human health assessment information on a chemical substance is included in the IRIS database only after a comprehensive review of toxicity data, as outlined in the IRIS assessment development process. Sections I (Health Hazard Assessments for Noncarcinogenic Effects) and II (Carcinogenicity Assessment for Lifetime Exposure) present the conclusions that were reached during the assessment development process. Supporting information and explanations of the methods used to derive the values given in IRIS are provided in the guidance documents located on the IRIS website. STATUS OF DATA FOR Methyl chlorocarbonate File First On-Line 08/22/1988 Category (section) Assessment Available? Last Revised Oral RfD (I.A.) withdrawn 05/01/1989* Inhalation RfC (I.B.) not evaluated Carcinogenicity Assessment (II.) not evaluated *A comprehensive review of toxicological studies was completed (2004) - please see section I.A. for more information. I. Chronic Health Hazard Assessments for Noncarcinogenic Effects I.A. Reference Dose for Chronic Oral Exposure (RfD) Substance Name — Methyl chlorocarbonate CASRN — 79-22-1 The RfD for methyl chlorocarbonate was withdrawn on 05/01/1989 pending further review by the RfD Work Group. A comprehensive review of toxicological studies published prior to 2004 indicated that there is insufficient health effects data to derive an RfD for Methyl chlorocarbonate at this time. 1 Integrated Risk Information System (IRIS) U.S. Environmental Protection Agency Chemical Assessment Summary National Center for Environmental Assessment EPA Contacts: Please contact the IRIS Hotline for all questions concerning this assessment or IRIS, in general, at (202)566-1676 (phone), (202)566-1749 (FAX) or [email protected] (internet address). -

Benzyl Chloroformate (CHLOROFORMIC ACID, BENZYL ESTER)
Rev B Benzyl Chloroformate (CHLOROFORMIC ACID, BENZYL ESTER) C8H7O2Cl Molecular Weight = 170.6 CAS# 501‐53‐1 SPECIFICATIONS Assay: 98.% min. Color (APHA): 50 max. Benzyl Alcohol: 0.1% max. Hydrogen Chloride: 0.1% max. Benzyl Chloride: 1.5% max. Phosgene: 0.1% max. Dibenzyl Carbonate: 0.5% max. Iron: 1.5 PPM max. PHYSICAL PROPERTIES Appearance: Clear liquid free of visible contaminants BP: Decomposes at elevated temperature Odor: Pungent Density: 1.195 ‐1.22 MP/Range: ‐30°C Flash Point: 126°C NOTICE: The technical information and suggestions for use made herein are based on VanDeMark’s research and experience and are believed to be reliable, but such information and suggestions do not constitute a warranty, and no patent liability can be assumed. This publication is not to be taken as a license to operate under or infringe on any patents. Since VanDeMark has no control over the conditions under which the product is transported, stored, handled, used or applied, buyer must determine for himself by preliminary tests or otherwise, the suitability of the product for his purposes. VanDeMark’s liability on any basis is limited to the price of the product used. The information in this bulletin supersedes all previously issued bulletins on the subject matter covered. VanDeMark Benzyl Chloroformate APPLICATIONS SPILLS AND DISPOSAL Benzyl Chloroformate is a reactive chemical intermediate Use personal protective equipment (see MSDS). used in the synthesis of pharmaceutical and agrochemical Evacuate personnel to safe areas. Dike far ahead of products. It is used as a reagent in peptide synthesis to liquid spill for later disposal. -

United States Patent Office Patented Nov
3,355,453 United States Patent Office Patented Nov. 28, 1967 1. 2 3,355,453 hydrogen chloride split off by the reaction, the amount of N-SUBSTITUTED BENZO-1,3-OXAZINE acid-binding agent present in the reaction mixture being at least one molar equivalent of the amount of chloro Klaus Hasspacher, BiberachDIGNE-(2,4) an der Riss, Germany, assigsb formic acid ester used as the starting material. Examples or, by messae assignments, to Boehringer Ingelhein of suitable acid-binding agents are inorganic bases, such G.m.b.H., Ingelheim am Rhein, Germany, a corpora tion of Germany as alkali metal hydroxides, alkali metal carbonates and No Drawing. Fied May 24, 1963, Ser. No. 282,887 the like, or tertiary organic bases, such as triethylamine, Claims priority, applicationa Germany, May 25, 1960, tributylamine, pyridine, N-methyl-piperidine and the like. T 18,445 The reaction is further advantageously performed in the 10 presence of an inert organic solvent, such as benzene, 12 Ciaims. (C. 269-244) toluene, tetrahydrofuran, dioxane and the like. These This is a continuation-in-part of copending applica inert organic solvents may also be present in the form of tion Ser. No. 111,926, filed May 23, 1961. mixtures with water, which is especially useful when an This invention relates to N-substituted benzo-1,3-ox inorganic base is used as the acid-binding agent. azine-diones-(2,4) and to various methods of preparing 5 The mixed anhydride Va obtained in the manner de these compounds. scribed above does not need to be isolated, but may in More particularly, the present invention relates to N stead be directly reacted with an amine of the Formula substituted benzo-1,3-oxazine-diones-(2,4) of the formula VI in the presence of one of the above mentioned solvents. -
O Y -C- -NH-COR4
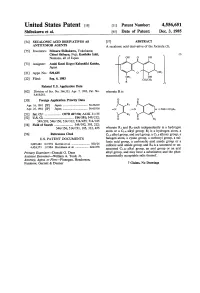
United States Patent (19) 11 Patent Number: 4,556,651 Shibukawa et al. 45 Date of Patent: Dec. 3, 1985 (54) SECALONIC ACID DERIVATIVES AS 57 ABSTRACT ANTITUMORAGENTS A secalonic acid derivative of the formula (I); (75) Inventors: Mitsuru Shibukawa, Yokohama; Chisei Shibuya, Fuji; Kunihiko Ishii, (I) Numazu, all of Japan 73) Assignee: Asahi Kasei Kogyo Kabushiki Kaisha, Japan (21) Appl. No.: 529,635 22 Filed: Sep. 6, 1983 Related U.S. Application Data 62 Division of Ser. No. 366,333, Apr. 7, 1982, Pat. No. wherein R is 4,418,061. 30 Foreign Application Priority Data Apr. 16, 1981 JP Japan .................................. 56-5629 R1 Apr. 25, 1981 JP Japan .................................. 56-61916 51) Int. Cl.' .................... C07D 407/04; A61K 31/35 - O R* y -C- -NH-COR4 52 U.S. C. .................................... 514/191; 549/212; O O R3 549/393; 546/156; 514/312; 514/455; 514/185 58 Field of Search ....................... 549/392,393, 212; 546/156; 514/191, 185, 312, 455 wherein R1 and R2 each independently is a hydrogen atom or a C1-4 alkyl group; R3 is a hydrogen atom, a 56) References Cited C1-4 alkyl group, and aryl group, a C1-5 alkoxy group, a U.S. PATENT DOCUMENTS halogen atom, a cyano group, a carboxyl group, a sul fonic acid group, a carboxylic acid amide group or a 3,829,463 8/1974 Kornis et al. ......................... 560/24 sulfonic acid amide group; and R4 is a saturated or un 4,424,373 1/1984 Kurobane et al. .................. 424/278 saturated C1-22 alkyl group, an aryl group or an aryl Primary Examiner-Donald G. -

Chlorine Oxidation of Vocs at a Semi-Rural Site in Beijing: Significant Chlorine Liberation from Clno2 and Subsequent Gas- and Particle-Phase Cl–VOC Production
Atmos. Chem. Phys., 18, 13013–13030, 2018 https://doi.org/10.5194/acp-18-13013-2018 © Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License. Chlorine oxidation of VOCs at a semi-rural site in Beijing: significant chlorine liberation from ClNO2 and subsequent gas- and particle-phase Cl–VOC production Michael Le Breton1, Åsa M. Hallquist2, Ravi Kant Pathak1, David Simpson3,4, Yujue Wang5, John Johansson3, Jing Zheng5, Yudong Yang5, Dongjie Shang5, Haichao Wang5, Qianyun Liu6, Chak Chan7, Tao Wang8, Thomas J. Bannan9, Michael Priestley9, Carl J. Percival9,a, Dudley E. Shallcross10,11, Keding Lu5, Song Guo5, Min Hu5, and Mattias Hallquist1 1Department of Chemistry and Molecular Biology, University of Gothenburg, Gothenburg, Sweden 2IVL Swedish Environmental Research Institute, Gothenburg, Sweden 3Earth and Space Sciences, Chalmers University of Technology, Gothenburg, Sweden 4Norwegian Meteorological Institute, Oslo, Norway 5State Key Joint Laboratory of Environmental Simulation and Pollution Control, College of Environmental Sciences and Engineering, Peking University, Beijing, China 6Division of Environment and Sustainability, Hong Kong University of Science and Technology, Clearwater Bay, Kowloon, Hong Kong 7School of Energy and Environment, City University of Hong Kong, Hong Kong 8Department of Civil and Environmental Engineering, Hong Kong Polytechnic University, Hong Kong 9Centre for Atmospheric Science, School of Earth, Atmospheric and Environmental Science, University of Manchester, Manchester, UK 10School of Chemistry, University of Bristol, Cantock’s Close, Bristol, UK 11Department of Chemistry, University of the Western Cape, Bellville, Cape Town, South Africa anow at: Jet Propulsion Laboratory, California Institute of Technology, 4800 Oak Grove Drive, Pasadena, CA, USA Correspondence: Michael Le Breton ([email protected]) Received: 9 January 2018 – Discussion started: 16 January 2018 Revised: 4 August 2018 – Accepted: 21 August 2018 – Published: 11 September 2018 Abstract. -

CAS Number Index 255
CASNumber Index CAS numbers of the substances listed in Sections II to XV and on the yellow pages CAS number Substance 50-00-0 Formaldehyde 50-29-3 DDT (Dichlorodiphenyltrichloroethane) 50-32-8 Benzo[a]pyrene (pyrolysis product) 50-53-3 Chlorpromazine (2-Chloro-10-(3-dimethylaminopropyl)phenothiazine) 51-75-2 N-Methyl-bis(2-chloroethyl)amine (nitrogen mustard) 51-79-6 Carbamic acid ethyl ester 52-51-7 2-Bromo-2-nitro-1,3-propanediol 53-70-3 Dibenz[a,h]anthracene (pyrolysis product) 54-11-5 Nicotine 54-64-8 Thimerosal 55-18-5 N-Nitrosodiethylamine 55-38-9 Fenthion 55-63-0 Nitroglycerin 56-23-5 Carbon tetrachloride 56-24-6 Trimethyltinhydroxide (TMTOH) (Methyltin compounds) 56-35-9 Bis(tributyltin)oxide (Tri-n-butyltin compounds) 56-38-2 Parathion 56-55-3 Benz[a]anthracene (pyrolysis product) 56-81-5 Glycerin 57-10-3 Palmitic acid 57-11-4 Stearic acid 57-14-7 1,1-Dimethylhydrazine 57-24-9 Strychnine 57-55-6 Propylene glycol 57-57-8 b-Propiolactone 57-74-9 Chlordane 58-89-9 Lindane (g-Hexachlorocyclohexane) 59-50-7 p-Chloro-m-cresol 59-89-2 N-Nitrosomorpholine 60-00-4 Ethylenediaminetetraacetic acid (EDTA) 60-09-3 p-Aminoazobenzene 60-12-8 2-Phenylethanol 60-29-7 Ethyl ether 60-34-4 Monomethylhydrazine 60-35-5 Acetamide 60-57-1 Dieldrin 61-82-5 Amitrole 62-23-7 4-Nitrobenzoic acid (yellow pages) 62-50-0 Methanesulfonic acid ethyl ester (yellow pages) 62-53-3 Aniline 62-56-6 Thiourea 62-73-7 Dichlorvos (DDVP) 62-74-8 Sodium fluoroacetate 62-75-9 N-Nitrosodimethylamine 63-25-2 Carbaryl 64-02-8 Ethylenediaminetetraacetic acid tetrasodium salt (Na EDTA) (yellow pages) 64-17-5 Ethanol 64-18-6 Formic acid 64-19-7 Aceticacid 64-67-5 Diethylsulfate List of MAK and BATValues 2015.DFG,Deutsche Forschungsgemeinschaft Copyright 2015 WILEY-VCH Verlag GmbH &Co. -
Potential Military Chemical/Biological Agents and Compounds (FM 3-11.9)
ARMY, MARINE CORPS, NAVY, AIR FORCE POTENTIAL MILITARY CHEMICAL/BIOLOGICAL AGENTS AND COMPOUNDS FM 3-11.9 MCRP 3-37.1B NTRP 3-11.32 AFTTP(I) 3-2.55 JANUARY 2005 DISTRIBUTION RESTRICTION: Approved for public release; distribution is unlimited. MULTISERVICE TACTICS, TECHNIQUES, AND PROCEDURES FOREWORD This publication has been prepared under our direction for use by our respective commands and other commands as appropriate. STANLEY H. LILLIE EDWARD HANLON, JR. Brigadier General, USA Lieutenant General, USMC Commandant Deputy Commandant US Army Chemical School for Combat Development JOHN M. KELLY BENTLEY B. RAYBURN Rear Admiral, USN Major General, USAF Commander Commander Navy Warfare Development Command Headquarters Air Force Doctrine Center This publication is available at Army Knowledge Online <www.us.army.mil>. PREFACE 1. Scope This document provides commanders and staffs with general information and technical data concerning chemical/biological (CB) agents and other compounds of military interest such as toxic industrial chemicals (TIC). It explains the use; classification; and physical, chemical, and physiological properties of these agents and compounds. Users of this manual are nuclear, biological, and chemical (NBC)/chemical, biological, and radiological (CBR) staff officers, NBC noncommissioned officers (NCOs), staff weather officers (SWOs), NBC medical defense officers, medical readiness officers, medical intelligence officers, field medical treatment officers, and others involved in planning battlefield operations in an NBC environment. 2. Purpose This publication provides a technical reference for CB agents and related compounds. The technical information furnished provides data that can be used to support operational assessments based on intelligence preparation of the battlespace (IPB). 3. Application The audience for this publication is NBC/CBR staff personnel and commanders tasked with planning, preparing for, and conducting military operations. -
Ethyl Chloroformate Hazard Summary Identification
Common Name: ETHYL CHLOROFORMATE CAS Number: 541-41-3 RTK Substance number: 0865 DOT Number: UN 1182 Date: March 2000 --------------------------------------------------------------------- --------------------------------------------------------------------- HAZARD SUMMARY * Ethyl Chloroformate can affect you when breathed in * Exposure to hazardous substances should be routinely and may be absorbed through the skin. evaluated. This may include collecting personal and area * Ethyl Chloroformate is a CORROSIVE CHEMICAL and air samples. You can obtain copies of sampling results contact can severely irritate and burn the skin and eyes from your employer. You have a legal right to this with possible eye damage. information under OSHA 1910.1020. * Breathing Ethyl Chloroformate can irritate the nose and * If you think you are experiencing any work-related health throat. problems, see a doctor trained to recognize occupational * Breathing Ethyl Chloroformate can irritate the lungs diseases. Take this Fact Sheet with you. causing coughing and/or shortness of breath. Higher exposures can cause a build-up of fluid in the lungs WORKPLACE EXPOSURE LIMITS (pulmonary edema), a medical emergency, with severe No occupational exposure limits have been established for shortness of breath. Ethyl Chloroformate. This does not mean that this substance * Exposure to Ethyl Chloroformate can cause headache, is not harmful. Safe work practices should always be nausea and vomiting. followed. * Repeated high exposure may affect the liver and kidneys. * Ethyl Chloroformate is a FLAMMABLE LIQUID and a * It should be recognized that Ethyl Chloroformate may be FIRE HAZARD. absorbed through your skin, thereby increasing your exposure. IDENTIFICATION Ethyl Chloroformate is a water-white liquid. It is used in the WAYS OF REDUCING EXPOSURE manufacture of ore flotation agents and in organic synthesis. -

United States Patent Office Patented July 19, 1960
. 2,945,860 United States Patent Office Patented July 19, 1960 2 formic acid carbethoxy-methyl ester, chloro-formic acid 2,945,860 butoxy-ethyl ester, chloro-formic acid chlorethyl ester, chloro-formic acid phenyl ester, chloro-formic acid NEW PIPERAZENE-CARBOXYLIC ACID ESTERs methoxy-phenyl ester, chloro-formic acid propoxy-phenyl AND PROCESS OF PREPARNG THEM ester, chloro-formic acid benzyloxy-phenyl ester, chloro Dieter Schmidt-Barbo, Hofheim (Taunus), Friedrich formic acid carbo-benzyloxyphenyl ester, chloro-formic Hampe, Bad Soden (Taunus), and Manfred Schorraid acid tolyl ester, chloro-formic acid nonyl-phenyl ester and Georg Lämmler, Frankfurt am Main, Germany, assig chloro-formic acid chloro-phenyl ester. ors to Farb werke Hoechst Aktiengesellschaft vormals The process according to the invention can be carried Meister Lucius & Bruning, Frankfurt an Main, Ger O -out by reacting 1-(3'-halogeno-4'-methyl-phenyl)piper many, a corporation of Germany azines with halogen-formic acid esters. In this case it is No Drawing. Filed Mar. 7, 1958, Ser, No. 719,757 advisable to operate in the presence of a solvent. As such there may for example be used hydrocarbons such Claims priority, application Germany Mar. 8, 1957 as benzine or benzene, halogen hydrocarbons such as 5 methylene chloride or chloroform, ethers such as diethyl 7 Claims. (CI. 260-268) - ether or di-isopropyl ether or ketones such as acetone. It is known that certain piperazine derivatives have In order to obtain the end products in good yields, it is of gained special importance for the control of blood para advantage to add basic compounds such as alkali metal sites. -

METHYL CHLOROFORMATE CAS Number
Common Name: METHYL CHLOROFORMATE CAS Number: 79-22-1 RTK Substance number: 1238 DOT Number: UN 1238 Date: June 1999 ----------------------------------------------------------------------- ----------------------------------------------------------------------- HAZARD SUMMARY * Methyl Chloroformate can affect you when breathed in. * Exposure to hazardous substances should be routinely * Methyl Chloroformate is a HIGHLY CORROSIVE evaluated. This may include collecting personal and area CHEMICAL and contact can severely irritate and burn the air samples. You can obtain copies of sampling results skin and eyes with possible eye damage. from your employer. You have a legal right to this * Breathing Methyl Chloroformate can irritate the nose information under OSHA 1910.1020. and throat. * If you think you are experiencing any work-related health * Breathing Methyl Chloroformate can irritate the lungs problems, see a doctor trained to recognize occupational causing coughing and/or shortness of breath. Higher diseases. Take this Fact Sheet with you. exposures can cause a build-up of fluid in the lungs (pulmonary edema), a medical emergency, with severe WORKPLACE EXPOSURE LIMITS shortness of breath. No occupational exposure limits have been established for * Methyl Chloroformate may cause a skin allergy. If Methyl Chloroformate. This does not mean that this allergy develops, very low future exposure can cause substance is not harmful. Safe work practices should always itching and a skin rash. be followed. * Methyl Chloroformate is a HIGHLY FLAMMABLE LIQUID and a DANGEROUS FIRE HAZARD. WAYS OF REDUCING EXPOSURE * Where possible, enclose operations and use local exhaust IDENTIFICATION ventilation at the site of chemical release. If local exhaust Methyl Chloroformate is a colorless liquid with an ventilation or enclosure is not used, respirators should be unpleasant, odor. -
United States Patent (19) L L 3,933,802 Ferrini Et Al
United States Patent (19) l l 3,933,802 Ferrini et al. (45) Jan. 20, 1976 54 SULPHAMOYLBENZOIC ACID AMDES Primary Examiner-Sam Rosen Assistant Examiner-D. W. Robinson (75) Inventors: Pier Giorgio Ferrini, Binningen; Attorney, Agent, or Firm-Joseph G. Kolodny, John J. Georges Haas; Alberto Rossi, both of Oberwil, all of Switzerland Maitner; Theodore O. Groeger 73) Assignee: Ciba-Geigy Corporation, Ardsley, 57 ABSTRACT N.Y. New 2-amino-5-sulphamoylbenzoic acid amide of the (22 Filed: May 22, 1974 general formula (21) Appl. No.: 472,333 (30) Foreign Application Priority Data May 28, 1973 Switzerland......................... 7643/73 Apr. 1, 1974 Switzerland......................... 4507174 (52) U.S. Cl............................... 260/239.7; 424/229 5 Int. Cl.............. C07D 237/00; C07D 239/00; C07D 241/00; CO7D 251/00 58) Field of Search......... 260/239.6, 239.7, 239.75, 260/239.65; 424/229 wherein R. denotes lower alkyl, R, denotes hydrogen, halogen, trifluoromethyl, lower alkyl or lower alkoxy, 56) References Cited Ra denotes hydrogen, hydroxyl or lower alkyl, R. de UNITED STATES PATENTS notes acyl and alk denotes lower 1,2-alkylene or 1,3- 2,910,488 0/959 Novello............................ 260f239.6 alkylene or a therapeutically acceptable acid addition 3,444,177 5/1969 Schmidt et al................... 260/239.7 salt thereof are usefull as mild analgesics with an anti FOREIGN PATENTS OR APPLICATIONS inflammatory component of action. 2,598 M 511963 France............................. 260f239.6 16 Claims, No Drawings 2,001,158 7|1970 Germany 3,933,802 1. and especially: butylene-1,2,w 2 propylene-1,2 and ethyl ene should be mentioned as examples.