Double-Brooding in Southern Yellow-Billed Hornbills Tockus Leucomelas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Smithsonian Miscellaneous Collections
SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 131, NUMBER 9 BREEDING AND OTHER HABITS OF CASQUED HORNBILLS (BYCANISTES SUBCYLINDRICUS) (With 6 Plates) By LAWRENCE KILHAM Bethesda, Md. (Publication 4259) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION NOVEMBER 8, 1956 THE LORD BALTIMORE PRESS, INC. BALTIMORE, MD., U. S. A. PREFACE I went to Uganda at the invitation of the East African High Com- mission to carry on virus research as a visiting scientist at the Virus Research Institute, Entebbe, where I worked from August 1954 until mid-May 1955. My ornithological observations were made as an ama- teur in the early mornings and evenings, and on weekends. It had been my hope to study some particular field problem in addition to making a general acquaintance with African bird life. The nature of the prob- lem was determined soon after my arrival. In my bird notes, black- and-white casqued hornbills [Bycanistes suhcylindricits (Sclater)] soon took up more pages than any other species. They came to our garden frequently. In addition, a pair of them roosted and carried on courtship activities in a tree above our house. When I discovered a concentration of hornbill nests in the Mpanga Research Forest, it was apparent that I had an unusual opportunity to study the natural history of casqued hornbills. Present studies did not begin until many females were already walled in. A few pairs of late-nesting hornbills, however, enabled me to witness the beginning stages of nesting ac- tivity. Observations on 16 nesting pairs gave, in the aggregate, a rounded picture of breeding and other habits of these birds. -

The Birds (Aves) of Oromia, Ethiopia – an Annotated Checklist
European Journal of Taxonomy 306: 1–69 ISSN 2118-9773 https://doi.org/10.5852/ejt.2017.306 www.europeanjournaloftaxonomy.eu 2017 · Gedeon K. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:A32EAE51-9051-458A-81DD-8EA921901CDC The birds (Aves) of Oromia, Ethiopia – an annotated checklist Kai GEDEON 1,*, Chemere ZEWDIE 2 & Till TÖPFER 3 1 Saxon Ornithologists’ Society, P.O. Box 1129, 09331 Hohenstein-Ernstthal, Germany. 2 Oromia Forest and Wildlife Enterprise, P.O. Box 1075, Debre Zeit, Ethiopia. 3 Zoological Research Museum Alexander Koenig, Centre for Taxonomy and Evolutionary Research, Adenauerallee 160, 53113 Bonn, Germany. * Corresponding author: [email protected] 2 Email: [email protected] 3 Email: [email protected] 1 urn:lsid:zoobank.org:author:F46B3F50-41E2-4629-9951-778F69A5BBA2 2 urn:lsid:zoobank.org:author:F59FEDB3-627A-4D52-A6CB-4F26846C0FC5 3 urn:lsid:zoobank.org:author:A87BE9B4-8FC6-4E11-8DB4-BDBB3CFBBEAA Abstract. Oromia is the largest National Regional State of Ethiopia. Here we present the first comprehensive checklist of its birds. A total of 804 bird species has been recorded, 601 of them confirmed (443) or assumed (158) to be breeding birds. At least 561 are all-year residents (and 31 more potentially so), at least 73 are Afrotropical migrants and visitors (and 44 more potentially so), and 184 are Palaearctic migrants and visitors (and eight more potentially so). Three species are endemic to Oromia, 18 to Ethiopia and 43 to the Horn of Africa. 170 Oromia bird species are biome restricted: 57 to the Afrotropical Highlands biome, 95 to the Somali-Masai biome, and 18 to the Sudan-Guinea Savanna biome. -

Ghana Comprehensive: Rockfowl & Upper Guinea Specials 11Th to 26Th November 2018 (16 Days) Trip Report
Ghana Comprehensive: Rockfowl & Upper Guinea Specials 11th to 26th November 2018 (16 days) Trip Report Black Bee-eater by Tuomas Seimola Trip report compiled by Tour Leader: Tuomas Seimola Rockjumper Birding Tours View more tours to Ghana Trip Report – RBL Ghana – Comprehensive 2018 2 Top 10 Birds 1. White-necked Rockfowl 6. Oriole Warbler 2. Black Bee-eater 7. Guinea/Yellow-billed Turacos 3. Egyptian Plover 8. Yellow Penduline Tit/White-crested Hornbill 4. Yellow-crowned Gonolek 9. Red-cheeked Wattle-eye 5. Violet Turaco 10. Red-billed Helmetshrike ___________________________________________________________________________________ Tour Summary Ghana is often described as the jewel of West Africa. This is not far from the truth. The diverse natural habitats combined with a tourist-friendly atmosphere and well-maintained road network make Ghana a real birder’s paradise. A visit to the World Heritage Site of Cape Coast Castle and understanding its controversial history was a powerful experience. The rainforest areas near Kakum National Park and vast savannas of Mole National Park were certainly highlights of this extraordinary tour. We tallied over 400 species of birds and over 20 mammals. These included highly sought- after gems like White-necked Rockfowl, Egyptian Plover, Blue-moustached Bee-eater, Akun Eagle-Owl, Stone Partridge, White- spotted Flufftail, Yellow-billed Turaco, Red- billed Dwarf Hornbill and many, many more. _____________________________________ The Tour in Detail Our first bird in Ghana was a fabulously performing Yellow-crowned Gonolek seen from the breakfast table – not a bad start! We spent the morning in Shai hills, which is Violet Turaco by Tuomas Seimola located north-east of the capital, Accra. -

Avibase Page 1Of 5
Avibase Page 1of 5 Col Location Date Start time Duration Distance Avibase - Bird Checklists of the World 1 Country or region: Gombe National Park 2 Number of species: 79 3 Number of endemics: 0 4 Number of breeding endemics: 0 5 Number of introduced species: 0 6 7 8 9 10 Recommended citation: Lepage, D. 2020. Checklist of the birds of Gombe National Park. Avibase, the world bird database. Retrieved from .https://avibase.bsc- eoc.org/checklist.jsp?lang=EN®ion=tzkm01&list=clements&format=1 [2/06/2020]. Make your observations count! Submit your data to ebird.org - Legend: [x] accidental [ex] extirpated [EX] extinct [EW] extinct in the wild [E] endemic [e] endemic (country/region) Common name Scientific name Synonym Status 1 2 3 4 5 6 7 8 9 10 GALLIFORMES: Numididae Helmeted Guineafowl Numida meleagris COLUMBIFORMES: Columbidae Laughing Dove Streptopelia senegalensis Emerald-spotted Wood-Dove Turtur chalcospilos Blue-spotted Wood-Dove Turtur afer Tambourine Dove Turtur tympanistria African Green-Pigeon Treron calvus CUCULIFORMES: Cuculidae Blue Malkoha Ceuthmochares aereus Pied Cuckoo Clamator jacobinus CAPRIMULGIFORMES: Apodidae Bat-like Spinetail Neafrapus boehmi African Palm-Swift Cypsiurus parvus CHARADRIIFORMES: Laridae White-winged Tern Chlidonias leucopterus CICONIIFORMES: Ciconiidae Marabou Stork Leptoptilos crumenifer Yellow-billed Stork Mycteria ibis Avibase Page 2of 5 Common name Scientific name Synonym Status 1 2 3 4 5 6 7 8 9 10 SULIFORMES: Anhingidae African Darter Anhinga rufa SULIFORMES: Phalacrocoracidae Great Cormorant -

Namibia & the Okavango
Pel’s Fishing Owl - a pair was found on a wooded island south of Shakawe (Jan-Ake Alvarsson) NAMIBIA & THE OKAVANGO 21 SEPTEMBER – 8 OCTOBER 2017 LEADER: STEVE BRAINE For most of the country the previous three years drought had been broken and although too early for the mi- grants we did however do very well with birding generally. We searched and found all the near endemics as well as the endemic Dune Lark. Besides these we also had a new write-in for the trip! In the floodplains after observing a wonderful Pel’s Fishing Owl we travelled down a side channel of the Okavango River to look for Pygmy Geese, we were lucky and came across several pairs before reaching a dried-out floodplain. Four birds flew out of the reedbeds and looked rather different to the normal weavers of which there were many, a closer look at the two remaining birds revealed a beautiful pair of Cuckoo Finches. These we all enjoyed for a brief period before they followed the other birds which had now disappeared into the reedbeds. Very strong winds on three of the birding days made birding a huge challenge to say the least after not finding the rare and difficult Herero Chat we had to make alternate arrangements at another locality later in the trip. The entire tour from the Hosea Kutako International Airport outside the capital Windhoek and returning there nineteen days later delivered 375 species. Out of these, four birds were seen only by the leader, a further three species were heard but not seen. -

Visual Fields in Hornbills: Precision-Grasping and Sunshades
Ibis (2004), 146, 18–26 Blackwell Publishing Ltd. Visual fields in hornbills: precision-grasping and sunshades GRAHAM R. MARTIN1* & HENDRI C. COETZEE2 1School of Biosciences, The University of Birmingham, Edgbaston, Birmingham B15 2TT, UK 2Ground Hornbill Research and Conservation Project, Private Bag X1644, Warmbaths, 0480, Republic of South Africa Retinal visual fields were determined in Southern Ground Hornbills Bucorvus leadbeateri and Southern Yellow-billed Hornbills Tockus leucomelas (Coraciiformes, Bucerotidae) using an ophthalmoscopic reflex technique. In both species the binocular field is relatively long and narrow with a maximum width of 30° occurring 40° above the bill. The bill tip projects into the lower half of the binocular field. This frontal visual field topography exhibits a number of key features that are also found in other terrestrial birds. This supports the hypothesis that avian visual fields are of three principal types that are correlated with the degree to which vision is employed when taking food items, rather than with phylogeny. However, unlike other species studied to date, in both hornbill species the bill intrudes into the binocular field. This intrusion of the bill restricts the width of the binocular field but allows the birds to view their own bill tips. It is suggested that this is associated with the precision-grasping feeding technique of hornbills. This involves forceps-like grasping and manipulation of items in the tips of the large decurved bill. The two hornbill species differ in the extent of the blind area perpendicularly above the head. Interspecific comparison shows that eye size and the width of the blind area above the head are significantly cor- related. -

African Gray Hornbill Class: Aves
Tockus nasutus nasutus African Gray Hornbill Class: Aves. Order: Coraciiformes. Family: Bucerotidae. Other names: Gray hornbill, hornbill Physical Description: Male—dun-colored with a central light stripe from hind neck to rump; head, throat, neck more or less gray with a broad white stripe over eye to nape; wing feathers and coverts (feathers covering the bases of the quills of the wings and tail) edged with whitish; outer tail feathers tipped white; underside creamy white; bill black with a splash of cream at base of upper mandible. Female—smaller, bill is colored red at tip, and greater part of the upper mandible is yellow. Hornbills are about 18 inches, males being larger than females. The wingspan of the gray hornbill is 7.5- 10 inches and weighs 5-9oz. Diet in the Wild: bird eggs and nestlings, insects, rodents, lizards, frogs, supplemented with small fruit and seeds especially during the dry season. Diet at the Zoo: Apples, papayas, grapes, hard-boiled eggs, mealworms, softbill diet, pinkie mice Habitat & Range: Sub-Saharan and Eastern Africa into Arabia, living in open woodlands and tree hollows Life Span: Up to 25 years Perils in the wild: Birds of prey, some carnivores, man, habitat destruction Physical Adaptations: Hornbills have huge, two-tiered beaks that cause the birds to appear top-heavy. The bill is long forming dexterous forceps. The cutting edges are serrated for breaking up food. The casque surmounting the bill is a narrow ridge that may reinforce the upper mandible. In spite of its heavy appearance, the structure is a light skin of keratin overlying a bony support. -

50 Common Birds of Jos and Central Nigeria the Year
This poster shows 50 of the most common species of bird found in Jos and the surrounding area, numbered roughly in order from the most common to the least common. It should be noted that populations of individual species can fluctuate from year to year and from place to place. Most of the species shown are regular visitors to urban gardens and can be seen without too much difficulty, although some of them can only be seen for part of 50 Common Birds of Jos and Central Nigeria the year. Hausa names are given, if known, although many species have several Hausa names. 1. Laughing Dove (Streptopelia senegalensis) 2. African Thrush (Turdus pelios) 3. Speckled Mousebird 4. Common Bulbul (Pycnonotus barbatus) 5. Red-cheeked Cordon-bleu 6. Scarlet-chested Sunbird (Chalcomitra 7. Village Weaver (Ploceus cucullatus) 8. Variable Sunbird 9. Yellow-billed Kite (Milvus aegyptius) Hausa: Kurciya Hausa: Tsebebe (Colius striatus) Hausa: Koje (Uraeginthus bengalus) Hausa: Asisita senegalensis) Hausa: Sha Kauci Hausa: Marai / Gado (Cinnyris venustus) Hausa: Shirwa 10. Western Grey Plantain-eater 11. Yellow-crowned Gonolek (Laniarius 12. Northern Black Flycatcher 13. African Paradise Flycatcher* 14. Grey-backed Camaroptera 15. Common Kestrel (Falco tinnunculus) 16. Pied Crow (Corvus albus) 17. Red-eyed Dove (Streptopelia 18. Snowy-crowned Robin-chat (Crinifer piscator) Hausa: Kulkulu barbarus) Hausa: Ɗan dufuwa (Melaenornis edolioides) (Terpsiphone viridis) (Camaroptera brevicaudata) Hausa: Karammata Hausa: Hankaka semitorquata) Hausa: Bolo / Kirkir / Wala (Cossypha niveicapilla) 19. Piapiac (Ptilostomus afer) 20. African Yellow White-eye (Zosterops 21. African Blue Flycatcher 22. Red-billed Firefinch Lagonosticta( 23. Adamawa Turtle Dove 24. Yellow-fronted Tinkerbird 25. -

South Africa Mega Birding III 5Th to 27Th October 2019 (23 Days) Trip Report
South Africa Mega Birding III 5th to 27th October 2019 (23 days) Trip Report The near-endemic Gorgeous Bushshrike by Daniel Keith Danckwerts Tour leader: Daniel Keith Danckwerts Trip Report – RBT South Africa – Mega Birding III 2019 2 Tour Summary South Africa supports the highest number of endemic species of any African country and is therefore of obvious appeal to birders. This South Africa mega tour covered virtually the entire country in little over a month – amounting to an estimated 10 000km – and targeted every single endemic and near-endemic species! We were successful in finding virtually all of the targets and some of our highlights included a pair of mythical Hottentot Buttonquails, the critically endangered Rudd’s Lark, both Cape, and Drakensburg Rockjumpers, Orange-breasted Sunbird, Pink-throated Twinspot, Southern Tchagra, the scarce Knysna Woodpecker, both Northern and Southern Black Korhaans, and Bush Blackcap. We additionally enjoyed better-than-ever sightings of the tricky Barratt’s Warbler, aptly named Gorgeous Bushshrike, Crested Guineafowl, and Eastern Nicator to just name a few. Any trip to South Africa would be incomplete without mammals and our tally of 60 species included such difficult animals as the Aardvark, Aardwolf, Southern African Hedgehog, Bat-eared Fox, Smith’s Red Rock Hare and both Sable and Roan Antelopes. This really was a trip like no other! ____________________________________________________________________________________ Tour in Detail Our first full day of the tour began with a short walk through the gardens of our quaint guesthouse in Johannesburg. Here we enjoyed sightings of the delightful Red-headed Finch, small numbers of Southern Red Bishops including several males that were busy moulting into their summer breeding plumage, the near-endemic Karoo Thrush, Cape White-eye, Grey-headed Gull, Hadada Ibis, Southern Masked Weaver, Speckled Mousebird, African Palm Swift and the Laughing, Ring-necked and Red-eyed Doves. -

Kingfishers to Mousebirds
3.8 Kingshers to mousebirds - Atlas of Birds uncorrected proofs Copyrighted Material Kingfishers to Mousebirds he orders featured on this spread include many of the planet’s most P Size of orders Trogoniformes: trogons R Teye-catching bird families. Some, such as kingfishers and rollers, Number of species in order Trogons make up a single family, the Trogonidae, are known for their dazzling plumage. Others, such as toucans and Percentage of total bird species which numbers seven genera, including the spectacular quetzals (Pharomachrus spp.) of hornbills, sport preposterously big bills. Though smaller species Coraciiformes South and Central America. Their weak feet are in some groups may superficially resemble songbirds, all have a 403 species unique among animals in having a heterodactyl number of key anatomical differences from the Passeriformes, and 4.1% toe arrangement: first and second toes facing none can sing. backwards; third and fourth toes forwards. They are colourful but retiring birds that These orders also share many features of their breeding behaviour, inhabit tropical forests worldwide – with the with the majority of families and species nesting in holes, and many greatest diversity in the Neotropics – and use performing flamboyant courtship displays. The exception to this rule Piciformes their short, broad bill to feed on insects and are the Coliiformes of Sub-Saharan Africa, which are neither colourful 403 species fruit, generally gleaned from the branches in 4.1% a brief fluttering flight. Trogons are typically nor cavity nesters – they build a simple cup-shaped nest in foliage – and have located by their soft, insistent call, given ) an evolutionary history that sets them apart from other near-passerines. -
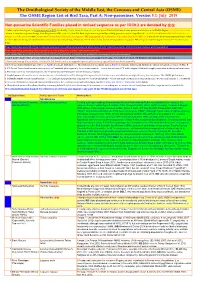
ORL 5.1 Non-Passerines Final Draft01a.Xlsx
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa, Part A: Non-passerines. Version 5.1: July 2019 Non-passerine Scientific Families placed in revised sequence as per IOC9.2 are denoted by ֍֍ A fuller explanation is given in Explanation of the ORL, but briefly, Bright green shading of a row (eg Syrian Ostrich) indicates former presence of a taxon in the OSME Region. Light gold shading in column A indicates sequence change from the previous ORL issue. For taxa that have unproven and probably unlikely presence, see the Hypothetical List. Red font indicates added information since the previous ORL version or the Conservation Threat Status (Critically Endangered = CE, Endangered = E, Vulnerable = V and Data Deficient = DD only). Not all synonyms have been examined. Serial numbers (SN) are merely an administrative convenience and may change. Please do not cite them in any formal correspondence or papers. NB: Compass cardinals (eg N = north, SE = southeast) are used. Rows shaded thus and with yellow text denote summaries of problem taxon groups in which some closely-related taxa may be of indeterminate status or are being studied. Rows shaded thus and with yellow text indicate recent or data-driven major conservation concerns. Rows shaded thus and with white text contain additional explanatory information on problem taxon groups as and when necessary. English names shaded thus are taxa on BirdLife Tracking Database, http://seabirdtracking.org/mapper/index.php. Nos tracked are small. NB BirdLife still lump many seabird taxa. A broad dark orange line, as below, indicates the last taxon in a new or suggested species split, or where sspp are best considered separately. -

African Hornbills: Keystone Species Threatened by Habitat Loss, Hunting and International Trade
Ostrich 2007, 78(3): 609–613 Copyright © NISC Pty Ltd Printed in South Africa — All rights reserved OSTRICH ISSN 0030–6525 doi: 10.2989/OSTRICH.2007.78.3.7.318 African hornbills: keystone species threatened by habitat loss, hunting and international trade Pepper W Trail US Fish and Wildlife Service, National Fish and Wildlife Forensics Laboratory, 1490 E Main Street, Ashland, OR 97520, USA e-mail: [email protected] Africa is home to 23 of the world’s 54 hornbill species, including the largest members of the family, the ground hornbills. None of Africa’s hornbills are currently considered to be at significant risk of extinction by IUCN, and none are listed under the Convention on International Trade in Endangered Species (CITES). However, there is evidence for serious declines of African forest hornbills due to habitat loss and fragmentation, and to unsustainable exploitation for bushmeat. In addition, this paper documents a previously unreported international trade involving importation of African hornbills and their parts into the United States. In the absence of CITES reporting requirements, it is difficult to estimate the magnitude of this trade, but it appears to represent an additional threat to African hornbills, particularly large forest-dwelling species of the genera Bycanistes and Ceratogymna. Given this international trade, and other known threats to African forest-dwelling hornbills, the status of these species is in urgent need of review. Introduction Hornbills are among the world’s most recognisable birds, with This lack of conservation concern probably reflects the many species exhibiting large body size, spectacular fact that all African hornbills are continental species with enlarged casques, striking black-and-white plumage, and extensive geographic ranges.