Impact Source Determination with Biomonitoring Data in New York State: Concordance with Environmental Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mohawk River Watershed – HUC-12
ID Number Name of Mohawk Watershed 1 Switz Kill 2 Flat Creek 3 Headwaters West Creek 4 Kayaderosseras Creek 5 Little Schoharie Creek 6 Headwaters Mohawk River 7 Headwaters Cayadutta Creek 8 Lansing Kill 9 North Creek 10 Little West Kill 11 Irish Creek 12 Auries Creek 13 Panther Creek 14 Hinckley Reservoir 15 Nowadaga Creek 16 Wheelers Creek 17 Middle Canajoharie Creek 18 Honnedaga 19 Roberts Creek 20 Headwaters Otsquago Creek 21 Mill Creek 22 Lewis Creek 23 Upper East Canada Creek 24 Shakers Creek 25 King Creek 26 Crane Creek 27 South Chuctanunda Creek 28 Middle Sprite Creek 29 Crum Creek 30 Upper Canajoharie Creek 31 Manor Kill 32 Vly Brook 33 West Kill 34 Headwaters Batavia Kill 35 Headwaters Flat Creek 36 Sterling Creek 37 Lower Ninemile Creek 38 Moyer Creek 39 Sixmile Creek 40 Cincinnati Creek 41 Reall Creek 42 Fourmile Brook 43 Poentic Kill 44 Wilsey Creek 45 Lower East Canada Creek 46 Middle Ninemile Creek 47 Gooseberry Creek 48 Mother Creek 49 Mud Creek 50 North Chuctanunda Creek 51 Wharton Hollow Creek 52 Wells Creek 53 Sandsea Kill 54 Middle East Canada Creek 55 Beaver Brook 56 Ferguson Creek 57 West Creek 58 Fort Plain 59 Ox Kill 60 Huntersfield Creek 61 Platter Kill 62 Headwaters Oriskany Creek 63 West Kill 64 Headwaters South Branch West Canada Creek 65 Fly Creek 66 Headwaters Alplaus Kill 67 Punch Kill 68 Schenevus Creek 69 Deans Creek 70 Evas Kill 71 Cripplebush Creek 72 Zimmerman Creek 73 Big Brook 74 North Creek 75 Upper Ninemile Creek 76 Yatesville Creek 77 Concklin Brook 78 Peck Lake-Caroga Creek 79 Metcalf Brook 80 Indian -

A Chronicle of the Wood Family
YORKSHIRE TO WESTCHESTER A CHRONICLE OF THE WOOD FAMILY By_ HERBERT BARBER HOWE PUBLISHED IN THE UNITED STATES OF AMERICA By THE TUTTLE PususHING Co., INC. · Edwin F. Sharp, Lessee RUTLAND, VERMONT 1948 ]AMES Woon 1762-1852 THIS BOOK IS DEDICATED TO THE COUSINS GRACE WOOD HAVILAND AND ELIZABETH RUNYON HOWE WHO OWN THE HOUSES BUILT BY THEIR GRANDFATHERS ON THE LAND ACQUIRED BY THEIR GREAT-GREAT-GRANDFATHER COUNTY OFFICE BUILDING WHITE PLAINS, NEW YORK The Westchester County Historical Society welcomes this volume as an important addition to the history of a rapidly changing country side. Mr. Howe, who is the highly effective editor of the Society's Bulletin, has been resourceful in his research and engaging in his presentation of his findings. Many a genealogical clue has been fol lowed to the point where an important discovery concerning persons or events was possible. Yet Mr. Howe is insistent that there are still loose ends in his study; for the absence of records can thwart the most determined chronicler. The story of the Wood family, from the days of the restless Puri tans in Yorkshire to the present era in Westchester, is richly furnished with exciting incidents and worthy achievements. The Tory an cestor during the American Revolution, who migrated to Nova Scotia and there built for himself a new life, is typical of many per sons in Westchester, who could not follow the signers of the Declara tion of Independence in their decision to break allegiance ro the British Empire. In the case of the Wood family the conflicting theories of empire brought a break in family ties during those troub lous years; and the task of the family historian was thus made much more difficult. -

Research Report110
~ ~ WISCONSIN DEPARTMENT OF NATURAL RESOURCES A Survey of Rare and Endangered Mayflies of Selected RESEARCH Rivers of Wisconsin by Richard A. Lillie REPORT110 Bureau of Research, Monona December 1995 ~ Abstract The mayfly fauna of 25 rivers and streams in Wisconsin were surveyed during 1991-93 to document the temporal and spatial occurrence patterns of two state endangered mayflies, Acantha metropus pecatonica and Anepeorus simplex. Both species are candidates under review for addition to the federal List of Endang ered and Threatened Wildlife. Based on previous records of occur rence in Wisconsin, sampling was conducted during the period May-July using a combination of sampling methods, including dredges, air-lift pumps, kick-nets, and hand-picking of substrates. No specimens of Anepeorus simplex were collected. Three specimens (nymphs or larvae) of Acanthametropus pecatonica were found in the Black River, one nymph was collected from the lower Wisconsin River, and a partial exuviae was collected from the Chippewa River. Homoeoneuria ammophila was recorded from Wisconsin waters for the first time from the Black River and Sugar River. New site distribution records for the following Wiscon sin special concern species include: Macdunnoa persimplex, Metretopus borealis, Paracloeodes minutus, Parameletus chelifer, Pentagenia vittigera, Cercobrachys sp., and Pseudiron centra/is. Collection of many of the aforementioned species from large rivers appears to be dependent upon sampling sand-bottomed substrates at frequent intervals, as several species were relatively abundant during only very short time spans. Most species were associated with sand substrates in water < 2 m deep. Acantha metropus pecatonica and Anepeorus simplex should continue to be listed as endangered for state purposes and receive a biological rarity ranking of critically imperiled (S1 ranking), and both species should be considered as candidates proposed for listing as endangered or threatened as defined by the Endangered Species Act. -

Charles River Watershed 2002 Biological Assessment
Technical Memorandum TM-72-8 CHARLES RIVER WATERSHED 2002 BIOLOGICAL ASSESSMENT John F. Fiorentino Massachusetts Department of Environmental Protection Division of Watershed Management 7 December 2005 CN 191.0 Charles River Watershed 2002-2006 Water Quality Assessment Report Appendix C C1 72wqar07.doc DWM CN 136.5 CONTENTS Introduction 3 Basin Description 6 Methods 7 Macroinvertebrate Sampling 7 Macroinvertebrate Sample Processing and Analysis 8 Habitat Assessment 9 Quality Control 9 Results and Discussion 10 Summary and Recommendations 26 Literature Cited 28 Appendix – Macroinvertebrate taxa list, RBPIII benthos analysis, Habitat evaluations 31 Tables and Figures Table 1. Macroinvertebrate biomonitoring station locations 4 Table 2. Perceived problems addressed during the 2002 survey 4 Table 3. Summary of possible causes of benthos impairment and recommended actions 27 Figure 1. Map showing biomonitoring station locations 5 Figure 2. DEP biologist conducting macroinvertebrate “kick” sampling 7 Figure 3. Schematic of the RBPIII analysis as it relates to Tiered Aquatic Life Use 26 Charles River Watershed 2002-2006 Water Quality Assessment Report Appendix C C2 72wqar07.doc DWM CN 136.5 INTRODUCTION Biological monitoring is a useful means of detecting anthropogenic impacts to the aquatic community. Resident biota (e.g., benthic macroinvertebrates, fish, periphyton) in a water body are natural monitors of environmental quality and can reveal the effects of episodic and cumulative pollution and habitat alteration (Barbour et al. 1999, Barbour et al. 1995). Biological surveys and assessments are the primary approaches to biomonitoring. As part of the Massachusetts Department of Environmental Protection/ Division of Watershed Management’s (MassDEP/DWM) 2002 Charles River watershed assessments, aquatic benthic macroinvertebrate biomonitoring was conducted to evaluate the biological health of various streams within the watershed. -

Biological Monitoring of Surface Waters in New York State, 2019
NYSDEC SOP #208-19 Title: Stream Biomonitoring Rev: 1.2 Date: 03/29/19 Page 1 of 188 New York State Department of Environmental Conservation Division of Water Standard Operating Procedure: Biological Monitoring of Surface Waters in New York State March 2019 Note: Division of Water (DOW) SOP revisions from year 2016 forward will only capture the current year parties involved with drafting/revising/approving the SOP on the cover page. The dated signatures of those parties will be captured here as well. The historical log of all SOP updates and revisions (past & present) will immediately follow the cover page. NYSDEC SOP 208-19 Stream Biomonitoring Rev. 1.2 Date: 03/29/2019 Page 3 of 188 SOP #208 Update Log 1 Prepared/ Revision Revised by Approved by Number Date Summary of Changes DOW Staff Rose Ann Garry 7/25/2007 Alexander J. Smith Rose Ann Garry 11/25/2009 Alexander J. Smith Jason Fagel 1.0 3/29/2012 Alexander J. Smith Jason Fagel 2.0 4/18/2014 • Definition of a reference site clarified (Sect. 8.2.3) • WAVE results added as a factor Alexander J. Smith Jason Fagel 3.0 4/1/2016 in site selection (Sect. 8.2.2 & 8.2.6) • HMA details added (Sect. 8.10) • Nonsubstantive changes 2 • Disinfection procedures (Sect. 8) • Headwater (Sect. 9.4.1 & 10.2.7) assessment methods added • Benthic multiplate method added (Sect, 9.4.3) Brian Duffy Rose Ann Garry 1.0 5/01/2018 • Lake (Sect. 9.4.5 & Sect. 10.) assessment methods added • Detail on biological impairment sampling (Sect. -

Freshwater Fishing: a Driver for Ecotourism
New York FRESHWATER April 2019 FISHINGDigest Fishing: A Sport For Everyone NY Fishing 101 page 10 A Female's Guide to Fishing page 30 A summary of 2019–2020 regulations and useful information for New York anglers www.dec.ny.gov Message from the Governor Freshwater Fishing: A Driver for Ecotourism New York State is committed to increasing and supporting a wide array of ecotourism initiatives, including freshwater fishing. Our approach is simple—we are strengthening our commitment to protect New York State’s vast natural resources while seeking compelling ways for people to enjoy the great outdoors in a socially and environmentally responsible manner. The result is sustainable economic activity based on a sincere appreciation of our state’s natural resources and the values they provide. We invite New Yorkers and visitors alike to enjoy our high-quality water resources. New York is blessed with fisheries resources across the state. Every day, we manage and protect these fisheries with an eye to the future. To date, New York has made substantial investments in our fishing access sites to ensure that boaters and anglers have safe and well-maintained parking areas, access points, and boat launch sites. In addition, we are currently investing an additional $3.2 million in waterway access in 2019, including: • New or renovated boat launch sites on Cayuga, Oneida, and Otisco lakes • Upgrades to existing launch sites on Cranberry Lake, Delaware River, Lake Placid, Lake Champlain, Lake Ontario, Chautauqua Lake and Fourth Lake. New York continues to improve and modernize our fish hatcheries. As Governor, I have committed $17 million to hatchery improvements. -
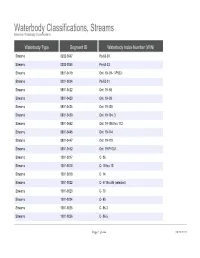
Waterbody Classifications, Streams Based on Waterbody Classifications
Waterbody Classifications, Streams Based on Waterbody Classifications Waterbody Type Segment ID Waterbody Index Number (WIN) Streams 0202-0047 Pa-63-30 Streams 0202-0048 Pa-63-33 Streams 0801-0419 Ont 19- 94- 1-P922- Streams 0201-0034 Pa-53-21 Streams 0801-0422 Ont 19- 98 Streams 0801-0423 Ont 19- 99 Streams 0801-0424 Ont 19-103 Streams 0801-0429 Ont 19-104- 3 Streams 0801-0442 Ont 19-105 thru 112 Streams 0801-0445 Ont 19-114 Streams 0801-0447 Ont 19-119 Streams 0801-0452 Ont 19-P1007- Streams 1001-0017 C- 86 Streams 1001-0018 C- 5 thru 13 Streams 1001-0019 C- 14 Streams 1001-0022 C- 57 thru 95 (selected) Streams 1001-0023 C- 73 Streams 1001-0024 C- 80 Streams 1001-0025 C- 86-3 Streams 1001-0026 C- 86-5 Page 1 of 464 09/28/2021 Waterbody Classifications, Streams Based on Waterbody Classifications Name Description Clear Creek and tribs entire stream and tribs Mud Creek and tribs entire stream and tribs Tribs to Long Lake total length of all tribs to lake Little Valley Creek, Upper, and tribs stream and tribs, above Elkdale Kents Creek and tribs entire stream and tribs Crystal Creek, Upper, and tribs stream and tribs, above Forestport Alder Creek and tribs entire stream and tribs Bear Creek and tribs entire stream and tribs Minor Tribs to Kayuta Lake total length of select tribs to the lake Little Black Creek, Upper, and tribs stream and tribs, above Wheelertown Twin Lakes Stream and tribs entire stream and tribs Tribs to North Lake total length of all tribs to lake Mill Brook and minor tribs entire stream and selected tribs Riley Brook -

New York Fishing Regulations Guide: 2017-18
NEW YORK Freshwater FISHING 2017–18 O cial Volume 9, Issue No. 1 April 2017 Regulations Guide Fishing Lake Champlain www.dec.ny.gov Most regulations are in eect April 1, 2017 through March 31, 2018 Message from the Governor New York: A State of Angling Opportunity If you are an angler, you know how lucky you are to live in New York. The Empire State is unmatched in the quality and diversity of our fish species, and ranks fifth in the nation for total acreage of freshwater fishing locations. There are so many opportunities to get out and enjoy this important sport, and I have made it a priority to ensure that all New Yorkers have access to the State’s abundant fishing resources. That is why the NY is Open for Fishing and Hunting initiative has provided more than $4 million over the last three years to rehabilitate existing boat launches and construct new launches across the state. In addition, New York continues to make great progress toward our goal of establishing 50 new or improved land and water access sites. In 2016, we opened new universally accessible fishing piers and hand carry launches on the Esopus Creek in Ulster County and Looking Glass Pond in Schoharie County. We refurbished the Dunkirk Harbor Fishing Pier in Chautauqua County, which now provides access for users of all abilities to the outstanding fishing found in this area of Lake Erie. 2017 will see the opening of a brand new boat launch on Meacham Lake in Franklin County and updated facility on the northern end of Cayuga Lake. -
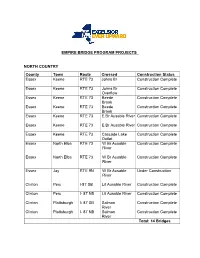
Empire Bridge Program Projects North Country
EMPIRE BRIDGE PROGRAM PROJECTS NORTH COUNTRY County Town Route Crossed Construction Status Essex Keene RTE 73 Johns Br Construction Complete Essex Keene RTE 73 Johns Br Construction Complete Overflow Essex Keene RTE 73 Beede Construction Complete Brook Essex Keene RTE 73 Beede Construction Complete Brook Essex Keene RTE 73 E Br Ausable River Construction Complete Essex Keene RTE 73 E Br Ausable River Construction Complete Essex Keene RTE 73 Cascade Lake Construction Complete Outlet Essex North Elba RTE 73 W Br Ausable Construction Complete River Essex North Elba RTE 73 W Br Ausable Construction Complete River Essex Jay RTE 9N W Br Ausable Under Construction River Clinton Peru I-87 SB Lit Ausable River Construction Complete Clinton Peru I- 87 NB Lit Ausable River Construction Complete Clinton Plattsburgh I- 87 SB Salmon Construction Complete River Clinton Plattsburgh I- 87 NB Salmon Construction Complete River Total: 14 Bridges CAPITAL DISTRICT County Town Route Crossed Construction Status Warren Thurman Rte 28 Hudson River Construction Complete Washington Hudson Falls Rte 196 Glens Falls Construction Complete Feeder Canal Washington Hudson Falls Rte 4 Glens Falls Construction Complete Feeder Saratoga Malta Rte 9 Kayaderosseras Construction Complete Creek Saratoga Greenfield Rte 9n Kayaderosseras Construction Complete Creek Rensselaer Nassau Rte 20 Kinderhook Creek Construction Complete Rensselaer Nassau Rte 20 Kinderhook Creek Construction Complete Rensselaer Nassau Rte 20 Kinderhook Creek Construction Complete Rensselaer Hoosick Rte -

Distribution of Ddt, Chlordane, and Total Pcb's in Bed Sediments in the Hudson River Basin
NYES&E, Vol. 3, No. 1, Spring 1997 DISTRIBUTION OF DDT, CHLORDANE, AND TOTAL PCB'S IN BED SEDIMENTS IN THE HUDSON RIVER BASIN Patrick J. Phillips1, Karen Riva-Murray1, Hannah M. Hollister2, and Elizabeth A. Flanary1. 1U.S. Geological Survey, 425 Jordan Road, Troy NY 12180. 2Rensselaer Polytechnic Institute, Department of Earth and Environmental Sciences, Troy NY 12180. Abstract Data from streambed-sediment samples collected from 45 sites in the Hudson River Basin and analyzed for organochlorine compounds indicate that residues of DDT, chlordane, and PCB's can be detected even though use of these compounds has been banned for 10 or more years. Previous studies indicate that DDT and chlordane were widely used in a variety of land use settings in the basin, whereas PCB's were introduced into Hudson and Mohawk Rivers mostly as point discharges at a few locations. Detection limits for DDT and chlordane residues in this study were generally 1 µg/kg, and that for total PCB's was 50 µg/kg. Some form of DDT was detected in more than 60 percent of the samples, and some form of chlordane was found in about 30 percent; PCB's were found in about 33 percent of the samples. Median concentrations for p,p’- DDE (the DDT residue with the highest concentration) were highest in samples from sites representing urban areas (median concentration 5.3 µg/kg) and lower in samples from sites in large watersheds (1.25 µg/kg) and at sites in nonurban watersheds. (Urban watershed were defined as those with a population density of more than 60/km2; nonurban watersheds as those with a population density of less than 60/km2, and large watersheds as those encompassing more than 1,300 km2. -

TB142: Mayflies of Maine: an Annotated Faunal List
The University of Maine DigitalCommons@UMaine Technical Bulletins Maine Agricultural and Forest Experiment Station 4-1-1991 TB142: Mayflies of aine:M An Annotated Faunal List Steven K. Burian K. Elizabeth Gibbs Follow this and additional works at: https://digitalcommons.library.umaine.edu/aes_techbulletin Part of the Entomology Commons Recommended Citation Burian, S.K., and K.E. Gibbs. 1991. Mayflies of Maine: An annotated faunal list. Maine Agricultural Experiment Station Technical Bulletin 142. This Article is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Technical Bulletins by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. ISSN 0734-9556 Mayflies of Maine: An Annotated Faunal List Steven K. Burian and K. Elizabeth Gibbs Technical Bulletin 142 April 1991 MAINE AGRICULTURAL EXPERIMENT STATION Mayflies of Maine: An Annotated Faunal List Steven K. Burian Assistant Professor Department of Biology, Southern Connecticut State University New Haven, CT 06515 and K. Elizabeth Gibbs Associate Professor Department of Entomology University of Maine Orono, Maine 04469 ACKNOWLEDGEMENTS Financial support for this project was provided by the State of Maine Departments of Environmental Protection, and Inland Fisheries and Wildlife; a University of Maine New England, Atlantic Provinces, and Quebec Fellow ship to S. K. Burian; and the Maine Agricultural Experiment Station. Dr. William L. Peters and Jan Peters, Florida A & M University, pro vided support and advice throughout the project and we especially appreci ated the opportunity for S.K. Burian to work in their laboratory and stay in their home in Tallahassee, Florida. -

SOP #: MDNR-WQMS-209 EFFECTIVE DATE: May 31, 2005
MISSOURI DEPARTMENT OF NATURAL RESOURCES AIR AND LAND PROTECTION DIVISION ENVIRONMENTAL SERVICES PROGRAM Standard Operating Procedures SOP #: MDNR-WQMS-209 EFFECTIVE DATE: May 31, 2005 SOP TITLE: Taxonomic Levels for Macroinvertebrate Identifications WRITTEN BY: Randy Sarver, WQMS, ESP APPROVED BY: Earl Pabst, Director, ESP SUMMARY OF REVISIONS: Changes to reflect new taxa and current taxonomy APPLICABILITY: Applies to Water Quality Monitoring Section personnel who perform community level surveys of aquatic macroinvertebrates in wadeable streams of Missouri . DISTRIBUTION: MoDNR Intranet ESP SOP Coordinator RECERTIFICATION RECORD: Date Reviewed Initials Page 1 of 30 MDNR-WQMS-209 Effective Date: 05/31/05 Page 2 of 30 1.0 GENERAL OVERVIEW 1.1 This Standard Operating Procedure (SOP) is designed to be used as a reference by biologists who analyze aquatic macroinvertebrate samples from Missouri. Its purpose is to establish consistent levels of taxonomic resolution among agency, academic and other biologists. The information in this SOP has been established by researching current taxonomic literature. It should assist an experienced aquatic biologist to identify organisms from aquatic surveys to a consistent and reliable level. The criteria used to set the level of taxonomy beyond the genus level are the systematic treatment of the genus by a professional taxonomist and the availability of a published key. 1.2 The consistency in macroinvertebrate identification allowed by this document is important regardless of whether one person is conducting an aquatic survey over a period of time or multiple investigators wish to compare results. It is especially important to provide guidance on the level of taxonomic identification when calculating metrics that depend upon the number of taxa.