Precuneus Shares Intrinsic Functional Architecture in Humans and Monkeys
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D
Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D. March 15, 2011 Anatomy Review • Lobes and layers • Brodmann’s areas • Vascular Supply • Major Neurological Findings – Frontal, Parietal, Temporal, Occipital, Limbic • Quiz Questions? Types of Cortex • Sensory • Motor • Unimodal association • Multimodal association necessary for language, reason, plan, imagine, create Structure of neocortex (6 layers) The general pattern of primary, association and mulimodal association cortex (Mesulam) Brodmann, Lateral Left Hemisphere MCA left hemisphere from D.Haines ACA and PCA -Haines Issues of Functional Localization • Earliest studies -Signs, symptoms and note location • Electrical discharge (epilepsy) suggested function • Ablation - deficit suggest function • Reappearance of infant functions suggest loss of inhibition (disinhibition), i.e. grasp, suck, Babinski • Variabilities in case reports • Linked networks of afferent and efferent neurons in several regions working to accomplish a task • Functional imaging does not always equate with abnormal function associated with location of lesion • fMRI activation of several cortical regions • Same sign from lesions in different areas – i.e.paraphasias • Notion of the right hemisphere as "emotional" in contrast to the left one as "logical" has no basis in fact. Limbic System (not a true lobe) involves with cingulate gyrus and the • Hippocampus- short term memory • Amygdala- fear, agression, mating • Fornix pathway to hypothalamus • -

10041.Full.Pdf
The Journal of Neuroscience, July 23, 2014 • 34(30):10041–10054 • 10041 Systems/Circuits Frontal Cortical and Subcortical Projections Provide a Basis for Segmenting the Cingulum Bundle: Implications for Neuroimaging and Psychiatric Disorders Sarah R. Heilbronner and Suzanne N. Haber Department of Pharmacology and Physiology, University of Rochester Medical Center, Rochester, New York 14642 The cingulum bundle (CB) is one of the brain’s major white matter pathways, linking regions associated with executive function, decision-making, and emotion. Neuroimaging has revealed that abnormalities in particular locations within the CB are associated with specific psychiatric disorders, including depression and bipolar disorder. However, the fibers using each portion of the CB remain unknown. In this study, we used anatomical tract-tracing in nonhuman primates (Macaca nemestrina, Macaca fascicularis, Macaca mulatta)toexaminetheorganizationofspecificcingulate,noncingulatefrontal,andsubcorticalpathwaysthroughtheCB.Thegoalswere as follows: (1) to determine connections that use the CB, (2) to establish through which parts of the CB these fibers travel, and (3) to relate the CB fiber pathways to the portions of the CB identified in humans as neurosurgical targets for amelioration of psychiatric disorders. Results indicate that cingulate, noncingulate frontal, and subcortical fibers all travel through the CB to reach both cingulate and noncin- gulate targets. However, many brain regions send projections through only part, not all, of the CB. For example, amygdala fibers are not present in the caudal portion of the dorsal CB. These results allow segmentation of the CB into four unique zones. We identify the specific connections that are abnormal in psychiatric disorders and affected by neurosurgical interventions, such as deep brain stimulation and cingulotomy. -

Brain Sulci and Gyri: a Practical Anatomical Review
Journal of Clinical Neuroscience 21 (2014) 2219–2225 Contents lists available at ScienceDirect Journal of Clinical Neuroscience journal homepage: www.elsevier.com/locate/jocn Neuroanatomical study Brain sulci and gyri: A practical anatomical review ⇑ Alvaro Campero a,b, , Pablo Ajler c, Juan Emmerich d, Ezequiel Goldschmidt c, Carolina Martins b, Albert Rhoton b a Department of Neurological Surgery, Hospital Padilla, Tucumán, Argentina b Department of Neurological Surgery, University of Florida, Gainesville, FL, USA c Department of Neurological Surgery, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina d Department of Anatomy, Universidad de la Plata, La Plata, Argentina article info abstract Article history: Despite technological advances, such as intraoperative MRI, intraoperative sensory and motor monitor- Received 26 December 2013 ing, and awake brain surgery, brain anatomy and its relationship with cranial landmarks still remains Accepted 23 February 2014 the basis of neurosurgery. Our objective is to describe the utility of anatomical knowledge of brain sulci and gyri in neurosurgery. This study was performed on 10 human adult cadaveric heads fixed in formalin and injected with colored silicone rubber. Additionally, using procedures done by the authors between Keywords: June 2006 and June 2011, we describe anatomical knowledge of brain sulci and gyri used to manage brain Anatomy lesions. Knowledge of the brain sulci and gyri can be used (a) to localize the craniotomy procedure, (b) to Brain recognize eloquent areas of the brain, and (c) to identify any given sulcus for access to deep areas of the Gyri Sulci brain. Despite technological advances, anatomical knowledge of brain sulci and gyri remains essential to Surgery perform brain surgery safely and effectively. -

Cuneus and Fusiform Cortices Thickness Is Reduced in Trigeminal
Parise et al. The Journal of Headache and Pain 2014, 15:17 http://www.thejournalofheadacheandpain.com/content/15/1/17 RESEARCH ARTICLE Open Access Cuneus and fusiform cortices thickness is reduced in trigeminal neuralgia Maud Parise1,2*, Tadeu Takao Almodovar Kubo1, Thomas Martin Doring1, Gustavo Tukamoto1, Maurice Vincent1,3 and Emerson Leandro Gasparetto1 Abstract Background: Chronic pain disorders are presumed to induce changes in brain grey and white matters. Few studies have focused CNS alterations in trigeminal neuralgia (TN). Methods: The aim of this study was to explore changes in white matter microstructure in TN subjects using diffusion tensor images (DTI) with tract-based spatial statistics (TBSS); and cortical thickness changes with surface based morphometry. Twenty-four patients with classical TN (37-67 y-o) and 24 healthy controls, matched for age and sex, were included in the study. Results: Comparing patients with controls, no diffusivity abnormalities of brain white matter were detected. However, a significant reduction in cortical thickness was observed at the left cuneus and left fusiform cortex in the patients group. The thickness of the fusiform cortex correlated negatively with the carbamazepine dose (p = 0.023). Conclusions: Since the cuneus and the fusiform gyrus have been related to the multisensory integration area and cognitive processing, as well as the retrieval of shock perception conveyed by Aδ fibers, our results support the role of these areas in TN pathogenesis. Whether such changes occurs as an epiphenomenon secondary to daily stimulation or represent a structural predisposition to TN in the light of peripheral vascular compression is a matter of future studies. -

Structure and Function of Visual Area MT
AR245-NE28-07 ARI 16 March 2005 1:3 V I E E W R S First published online as a Review in Advance on March 17, 2005 I E N C N A D V A Structure and Function of Visual Area MT Richard T. Born1 and David C. Bradley2 1Department of Neurobiology, Harvard Medical School, Boston, Massachusetts 02115-5701; email: [email protected] 2Department of Psychology, University of Chicago, Chicago, Illinois 60637; email: [email protected] Annu. Rev. Neurosci. Key Words 2005. 28:157–89 extrastriate, motion perception, center-surround antagonism, doi: 10.1146/ magnocellular, structure-from-motion, aperture problem by HARVARD COLLEGE on 04/14/05. For personal use only. annurev.neuro.26.041002.131052 Copyright c 2005 by Abstract Annual Reviews. All rights reserved The small visual area known as MT or V5 has played a major role in 0147-006X/05/0721- our understanding of the primate cerebral cortex. This area has been 0157$20.00 historically important in the concept of cortical processing streams and the idea that different visual areas constitute highly specialized Annu. Rev. Neurosci. 0.0:${article.fPage}-${article.lPage}. Downloaded from arjournals.annualreviews.org representations of visual information. MT has also proven to be a fer- tile culture dish—full of direction- and disparity-selective neurons— exploited by many labs to study the neural circuits underlying com- putations of motion and depth and to examine the relationship be- tween neural activity and perception. Here we attempt a synthetic overview of the rich literature on MT with the goal of answering the question, What does MT do? www.annualreviews.org · Structure and Function of Area MT 157 AR245-NE28-07 ARI 16 March 2005 1:3 pathway. -

A Role for the Left Angular Gyrus in Episodic Simulation and Memory
8142 • The Journal of Neuroscience, August 23, 2017 • 37(34):8142–8149 Behavioral/Cognitive A Role for the Left Angular Gyrus in Episodic Simulation and Memory X Preston P. Thakral, XKevin P. Madore, and XDaniel L. Schacter Department of Psychology, Harvard University, Boston, Massachusetts 02138 Functional magnetic resonance imaging (fMRI) studies indicate that episodic simulation (i.e., imagining specific future experiences) and episodic memory (i.e., remembering specific past experiences) are associated with enhanced activity in a common set of neural regions referred to as the core network. This network comprises the hippocampus, medial prefrontal cortex, and left angular gyrus, among other regions. Because fMRI data are correlational, it is unknown whether activity increases in core network regions are critical for episodic simulation and episodic memory. In the current study, we used MRI-guided transcranial magnetic stimulation (TMS) to assess whether temporary disruption of the left angular gyrus would impair both episodic simulation and memory (16 participants, 10 females). Relative to TMS to a control site (vertex), disruption of the left angular gyrus significantly reduced the number of internal (i.e., episodic) details produced during the simulation and memory tasks, with a concomitant increase in external detail production (i.e., semantic, repetitive, or off-topic information), reflected by a significant detail by TMS site interaction. Difficulty in the simulation and memory tasks also increased after TMS to the left angular gyrus relative to the vertex. In contrast, performance in a nonepisodic control task did not differ statistically as a function of TMS site (i.e., number of free associates produced or difficulty in performing the free associate task). -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Functional Connectivity of the Angular Gyrus in Normal Reading and Dyslexia (Positron-Emission Tomography͞human͞brain͞regional͞cerebral)
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 8939–8944, July 1998 Neurobiology Functional connectivity of the angular gyrus in normal reading and dyslexia (positron-emission tomographyyhumanybrainyregionalycerebral) B. HORWITZ*†,J.M.RUMSEY‡, AND B. C. DONOHUE‡ *Laboratory of Neurosciences, National Institute on Aging, and ‡Child Psychiatry Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892 Communicated by Robert H. Wurtz, National Eye Institute, Bethesda, MD, May 14, 1998 (received for review January 19, 1998) ABSTRACT The classic neurologic model for reading, not functionally connected during a specific task. On the other based on studies of patients with acquired alexia, hypothesizes hand, if rCBF in two regions is correlated, these regions need functional linkages between the angular gyrus in the left not be anatomically linked; their activities may be correlated, hemisphere and visual association areas in the occipital and for example, because both receive inputs from a third area (for temporal lobes. The angular gyrus also is thought to have more discussion about these connectivity concepts, see refs. 8, functional links with posterior language areas (e.g., Wer- 10, and 11). nicke’s area), because it is presumed to be involved in mapping Based on lesion studies in many patients with alexia, it has visually presented inputs onto linguistic representations. Us- been proposed that the posterior portion of the neural network ing positron emission tomography , we demonstrate in normal mediating reading in the left cerebral hemisphere involves men that regional cerebral blood flow in the left angular gyrus functional links between the angular gyrus and extrastriate shows strong within-task, across-subjects correlations (i.e., areas in occipital and temporal cortex associated with the functional connectivity) with regional cerebral blood flow in visual processing of letter and word-like stimuli (12–14). -
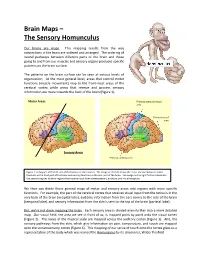
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Atrial Septal Defect (ASD) – Disorder of the Heart That Is Present at Birth Involving a Hole in the Wall (Septum) Separating the Two Upper Chambers (Atria)
Glossary of medical terms (grouped by affected system or organ) Atrial septal defect (ASD) – disorder of the heart that is present at birth involving a hole in the wall (septum) separating the two upper chambers (atria) Ventricular septal defect (VSD) – disorder of the heart that is present at birth involving a hole in the wall (septum) separating the two lower chambers (ventricles) Patent ductus arteriosus (PDA) – failure of the ductus arteriosus, an arterial shunt in fetal life, to close before birth (patent refers to remaining open) Polyvalvular disease – damage or defect to a heart valve (mitral, aortic, tricuspid or pulmonary); mitral and tricuspid valves control the flow of blood between the atria and the ventricles Pulmonary stenosis – narrowing or obstruction to blood flow (stenosis) from the right ventricle to the pulmonary artery Coarctation of aorta – narrowing of the aorta, the large blood vessel that delivers oxygen-rich blood to the body Bicuspid aortic valve - aortic valve separates the lower left chamber (left ventricle) of the heart from the aorta. A bicuspid aortic valve has two flaps (cusps) instead of the usual three. This condition is often present with coarctation of aorta. Mitral valve atresia – mitral valve connects the two chambers on the left side of the heart (atrium and ventricle). In this condition, blood is unable to flow between the two chambers. Hypoplastic aorta (hypoplastic left heart syndrome) – left side of the heart is critically underdeveloped so unable to effectively pump blood to the body and causing the right side of the heart to pump blood to the lungs and body. -

Apparent Atypical Callosal Dysgenesis: Analysis of MR Findings in Six Cases and Their Relationship to Holoprosencephaly
333 Apparent Atypical Callosal Dysgenesis: Analysis of MR Findings in Six Cases and Their Relationship to Holoprosencephaly A. James Barkovich 1 The MR scans of six pediatric patients with apparent atypical callosal dysgenesis (presence of the dorsal corpus callosum in the absence of a rostral corpus callosum) were critically analyzed and correlated with developmental information in order to assess the anatomic, embryologic, and developmental implications of this unusual anomaly. Four patients had semilobar holoprosencephaly; the dorsal interhemispheric commis sure in these four infants resembled a true callosal splenium. All patients in this group had severe developmental delay. The other two patients had complete callosal agenesis with an enlarged hippocampal commissure mimicking a callosal splenium; both were developmentally and neurologically normal. The embryologic implications of the pres ence of these atypical interhemispheric connections are discussed. Differentiation between semilobar holoprosencephaly and agenesis of the corpus callosum with enlarged hippocampal commissure-two types of apparent atypical callosal dysgenesis-can be made by obtaining coronal, short TR/TE MR images through the frontal lobes. Such differentiation has critical prognostic implications. AJNR 11:333-339, March{Apri11990 Abnormalities of the corpus callosum are frequently seen in patients with con genital brain malformations [1-5); a recent publication [5) reports an incidence of 47%. The corpus callosum normally develops in an anterior to posterior direction. The genu forms first, followed by the body, splenium, and rostrum. Dysgenesis of the corpus callosum is manifested by the presence of the earlier-formed segments (genu , body) and absence of the later-formed segments (splenium, rostrum) [4-6]. We have recently encountered six patients with findings suggestive of atypical callosal dysgenesis in whom there was apparent formation of the callosal splenium in the absence of the genu and body. -

Anatomy of the Corpus Callosum Reveals Its Function
The Journal of Neuroscience, February 13, 2008 • 28(7):1535–1536 • 1535 Journal Club Editor’s Note: These short critical reviews of recent papers in the Journal, written exclusively by graduate students or postdoctoral fellows, are intended to summarize the important findings of the paper and provide additional insight and comentary. For more information on the format and purpose of the Journal Club, please see http://www.jneurosci.org/misc/ifa_features.shtml. Anatomy of the Corpus Callosum Reveals Its Function Eric Mooshagian Department of Psychology, University of California, Los Angeles, California 90095-1563 Review of Wahl et al. (http://www.jneurosci.org/cgi/content/full/27/45/12132) The corpus callosum (CC) comprises ax- views of the CC. In contrast, a few recent amining callosal topography, suggesting a ons connecting the cortices of the two ce- studies have used diffusion tensor imag- more posterior crossing of CMFs (for dis- rebral hemispheres and is the principal ing (DTI) methods to re-evaluate callosal cussion, see Wahl et al., 2007). In addi- white matter fiber bundle in the brain. As topography (for discussion, see Wahl et tion, the present study goes beyond a recently as the mid 20th century, the CC al., 2007). These methods challenge the demonstration of topography by reveal- was thought to serve no other purpose conventional partitioning schemes used ing, for the first time, a clear somatotopy than preventing the two hemispheres to divide the CC into functionally signifi- of CMFs; hand fibers were situated ventral from collapsing on one another (Bogen, cant regions (Witelson, 1989). and anterior to foot fibers, and lip fibers, 1979).