Effects of a Fixed-Dose Combination Strategy on Adherence and Risk Factors in Patients with Or at High Risk of CVD the UMPIRE Randomized Clinical Trial
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GOVERNMENT of TELANGANA ABSTRACT Public Services
GOVERNMENT OF TELANGANA ABSTRACT Public Services – Formation /Reorganization of New Districts, Revenue Divisions and Mandals in Telangana State – Re-organization of Circles/Divisions/Sub- Divisions/Mandals in all cadres - Orders – Issued. PANCHAYAT RAJ & RURAL DEVELOPMENT (PR.I) DEPARTENT G.O.Ms.No.71 Dt:11.10.2016 Read the following:- 1. G.O.Ms.No.5, PR&RD(Estt.I) Dept. Dt:16.01.2015 and subsequent amendments, G.O.Ms.No.45, dt:23.5.2015, G.O.Ms.No.59, dt:31.7.2015 and G.O.Ms.No.6, dt:13.01.2016. 2. G.O.Ms.No.221 to 250, Revenue (DA-CMRF) Department, dt:11.10.2016 3. G.O.Ms.No.144, Finance (HRM.I) Department, dt:11.10.2016 4. From the E-in-C, PR, Hyderbad Letter No.B-II/Reorg.district/ 338/2016, Dt.17.9.2016, Dt:29.9.2016 & Dt:08.10.2016. ORDER: In the reference first read above Government have issued orders rationalising the PRI, PIU & Q C wings for effective implementation of works programme in PRED to achieve the targets of the Govt. 2. In the reference second read above Government of Telangana have issued notifications for formation/reorganization of Districts, Divisions and Mandals in the State of Telangana for better administration and development of areas concerned. 3. In the reference 3rd read above, Government have issued orders re- distributing cadre strength among (30) districts. 4. In the reference fourth read above the Engineer-in-Chief, PR has submitted proposals for re-organization of PRED to be co-terminus with the new districts jurisdiction and to change the nomenclature of Superintending Engineer, PR as Regional officer and Executive Engineer of the District Office as District Panchayat Raj Engineer (DPRE). -

Fairs and Festivals, (17 Karimnagar)
PRG. 179.17 (N) 750 KARIMNAGAR CENSUS OF INDIA 1961 VOLUME II ANDHRA PRADESH PART VII - B (17) F AIRS AND FESTIV (17. Karimnagar District) A. CHANDRA SEKHAR OF THE INDIAN ADMINISTRATIVE SERVICE Superintendent of Census Operations, Andhra Pradesh Price: Rs. 5.25 P. or 12 Sh. 3 d. or $ 1.89 c. 1961 CENSUS PUBLICATIONS, ANDHRA PRADESH (All the Census Publications of this State bear Vol. No. II) PART I-A (i) General Report (Chapters I to V) PART I-A (ii) General Report (Chapters VI to IX) PART I-A (iii) Gen'eral Report (Chapters eX to Xll) PART I-B Report on Vital Statistics PART I-C Subsidiary Tables PART II-A General Population Tables PART II-B (i) Economic Tables (B-1 to B-IV) PART II-B eii) Economic Tables (B-V to B-IX] PART II-C Cultural and Migration Tables PART III Household Economic Tables PART IV-A Report on Housing and Establishments (with Subsidiary Tables) PART IV-B Housing and Establishment Tables PART V-A Special Tables for Scheduled Castes and Scheduled Tribes PART V-B Ethnographic Notes on Scheduled Castes and Scheduled Tribes PART VI Villag~ Survey Monographs (46) PART VII-A (1) l PART VlI-A (2) ~ ... Handicrafts Survey Reports (Selected Crafts) I PART VII-A (3) J PART VII-B (1 to 20) ... Fairs and Festivals (Separate Book for each District) PART VIII-A Administration Report-Enumeration l }- (Not for sale) PART VIII-B Administration Report-Tabulation J PART IX State Atlas PART X Special Report on Hyderabad City District Census Handbooks (Separate Volume for each District) I 1. -

Salient Features of Time Table Coming Into Force from 01-7
SALIENT FEATURES OF TIME TABLE COMING INTO FORCE FROM 01.07.2012. I. INTRODUCTION OF NEW TRAINS (a) S.C.R. Trains: 1) Train No.12774/12773 Secunderabad – Shalimar-Secunderabd (Weekly) via Kazipet, Vijayawada – AC Express. Abstract timings: T.No. 12774 T.No.12773 (Secunderabd – Station (Shalimar - Shalimar) Secunderabad) 05.30 D Secunderabad A 19.00 07.45/07.50 a/d Waragal a/d 15.20/15.25 11.10/11.25 a/d Vijayawada a/d 12.10/12.30 13.36/13.38 a/d Rajhmundry a/d 09.20/09.22 14.17 d Samalkot d 08.26 17.25/17.45 a/d Visakhapatnam a/d 05.40/06.15 09.20 A Shalimar D 16.05 Days of service: Weekly Ex- Secunderabad : Tue Ex- Shalimar : Wed Commercial stoppages: Warangal,Vijayawada,Rajahmundry,Samalkot Jn. Visakhlapatnam Vizianagaram Srikakulam Road, Brahmapur, Kurda Road Jn. Bhubaneswar, Cuttack, Balasore, Kharagpur, Santragachi. Note: Date of Introduction will be notified later 2) Train No.12776/12775 Secunderabad – Kakinada-Secunderabad (Tri-Weekly) via Kazipet, Vijayawad, Gudivada, BVRT –AC Express Abstract timings: T.No.12776 T.No.12775 (Secunderabad – Station (Kakinada- Kakinada) Secunderabad) 21.15 D Secunderabad A 08.50 23.28/23.30 a/d Warangal a/d 05.39/05.40 01.04/01.05 a/d Khammam a/d 04.09/04.10 02.45/03.00 a/d Vijayawada a/d 02.45/03.00 03.53 d Gudivada d 01.52 04.39 d Akividu d 00.56 05.08 d Bhimavaram Town d 00.35 05.42 d Tanuku d 23.57 06.38/06.40 a/d Rajahmundry a/d 23.11/23.13 07.45 A Kakinada D 22.15 Days of service: Tri-weekly Ex-Secunderabadi : Mon, Wed, Fri Ex-Kakinada : Tue, Thu, Sun Commercial Stoppages: Warangal, Khammam, Vijayawada, Gudivada, Akividu, Bhimavaram Town, Tanuku, Rajahmundry Note: Date of Introduction will be notified later. -

Details of Staff Working at Dist. / Constituency / Mandal Level As on 27-07-2019
GOVERNMENT OF TELANGANA DEPARTMENT OF HORTICULTURE & SERICULTURE Details of Staff Working at Dist. / Constituency / Mandal Level as on 27-07-2019 INDEX Page Numbers Page Numbers S.No District Name S.No District Name From -- To From -- To 1 Adilabad 1 to 2 17 Mahabubnagar 29 to 30 2 Nirmal 3 to 4 18 Narayanapet 31 3 Mancherial 5 to 6 19 Nagarkurnool 32 to 33 4 Komarambheem 7 20 Gadwal 34 to 35 5 Karimnagar 8 to 9 21 Wanaparthy 36 to 37 6 Peddapalli 10 22 Vikarabad 38 to 39 7 Jagityal 11 to 12 23 Rangareddy 40 to 41 8 Siricilla 13 24 Medchal 42 9 Warangal ( R) 14 to 15 25 Sangareddy 43 to 44 10 Warangal(U) 16 to 17 26 Medak 45 to 46 11 Bhupalapally 18 to 19 27 Siddipiet 47 to 48 12 Mulugu 20 28 Nizamabad 49 to 50 13 Mahbubabad 21 to 22 29 Kamareddy 51 to 52 14 Jangaon 23 to 24 30 Nalgonda 53 to 55 15 Khammam 25 to 26 31 Suryapet 56 to 57 16 Kothagudem 27 to 28 32 Yadadri 58 to 59 Statement showing the Officer & Staff working in Horticulture & Sericulture Department No. of Assembly Constituencies : 2 Adilabad Constituency = 5 mandals Boath Constituency = 9 mandals Name of the new District:- No. of Mandals : 18 ADILABAD Part of Khanapur Constituency = 2 mandals Part of Asifabad constituency = 2 mandals Total mandals = 18 Sl. Name of the Employee Head Quarters / Assembly No. of Name of the Designation Name of the Mandals (Jurisdiction) No. Sarvasri/ Smt./ Kum. Constituency Mandals MLH&SO A - District Level Horticulture & Sericulture Officer Mulug, Venkatapur, Govindaraopet, K.Venkateshwarlu PD/ DH&SO, Adilabad 1 Adilabad Tadvai, Eturnagaram, Mangapet, 7997724995 (DDO - Adilabad ) Kannaigudem, Wajedu, Venkatapuram B - Constituency Level Officers Adilabad 1 Adilabad (R), Ch.Pranay Reddy MLH&SO(MIP) G.Srinivas Boath 1 Bheempur 7997725008 1 HO(T)/ CLH&SO 7997725002 A. -
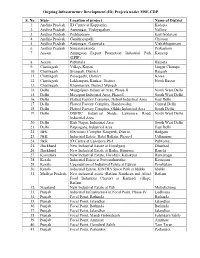
Ongoing Infrastructure Development (ID) Projects Under MSE-CDP
Ongoing Infrastructure Development (ID) Projects under MSE-CDP S. No. State Location of project Name of District 1. Andhra Pradesh ID Centre at Kopparthy Kadapa 2. Andhra Pradesh Autonagar, Vedayapalem Nellore 3. Andhra Pradesh Peddapuram East Godavari 4. Andhra Pradesh Gandhrajupalli Chittoor 5. Andhra Pradesh Autonagar, Gajuwaka Vishakhapatnam 6. Andhra Pradesh Singarayakonda Prakasham 7. Assam Amingaon Export Promotion Industrial Park Kamrup (EPIP) 8. Assam Pathshala Barpeta 9. Chattisgarh Village Kapan Janjgir Champa 10. Chattisgarh Siyarpali, District Raigarh 11. Chattisgarh Parasgadhi, District Korea 12. Chattisgarh Lakhanpuri, Kanker, District North Bastar 13. Chattisgarh Khamhariya, District Mungeli 14. Delhi Mangolpuri Industrial Area, Phase-II North West Delhi 15. Delhi Mayapuri Industrial Area, Phase-I South West Delhi 16. Delhi Flatted Factory Complex, Jhilmil Industrial Area East Delhi 17. Delhi Flatted Factory Complex, Jhandewalan Central Delhi 18. Delhi Flatted Factory Complex, Okhla Industrial Area South Delhi 19. Delhi DSIIDC Industrial Sheds, Lawrence Road, North West Delhi Industrial Area 20. Delhi Kirti Nagar, Industrial Area South West Delhi 21. Delhi Patparganj, Industrial Area East Delhi 22. J&K Electronic Complex Rangreth, District Badgam 23. J&K Industrial Estate, Batal Ballain, Phase-I Udhampur 24. J&K ID Centre at Lassipora Ph-I Pulwama 25. Jharkhand New Industrial Estate at Gopalganj Dhanbad 26. Jharkhand New Industrial Estate at Barhe, Bijupara Ranchi 27. Karnataka New Industrial Estate, Harohali, Kanakpur Ramanagar 28. Kerala Industrial Estate at Poovanthuruthu Kottayam 29. Kerala Upgradation of Industrial Estate at Edayar Ernakulam 30. Kerala Industrial Estate, KINFRA Spice Park at Idukki Idukki 31. Madhya Pradesh New industrial estate (Ratlam Namkeen and Allied Ratlam Food Industries Cluster) at Karmadi village, Ratlam 32. -

Karimnagar District, Andhra Pradesh
For Official Use Only CENTRAL GROUND WATER BOARD MINISTRY OF WATER RESOURCES GOVERNMENT OF INDIA GROUND WATER BROCHURE KARIMNAGAR DISTRICT, ANDHRA PRADESH SOUTHERN REGION HYDERABAD September 2013 CENTRAL GROUND WATER BOARD MINISTRY OF WATER RESOURCES GOVERNMENT OF INDIA GROUND WATER BROCHURE KARIMNAGAR DISTRICT, ANDHRA PRADESH (AAP-2012-13) BY R.V.V. SAGAR SCIENTIST-D SOUTHERN REGION BHUJAL BHAWAN, GSI Post, Bandlaguda NH.IV, FARIDABAD -121001 Hyderabad-500068 HARYANA, INDIA Andhra Pradesh Tel: 0129-2418518 Tel: 040-24225201 Gram: Bhumijal Gram: Antarjal GROUND WATER BROCHURE KARIMNAGAR DISTRICT, ANDHRA PRADESH CONTENTS DISTRICT AT A GLANCE 1. INTRODUCTION 2. RAINFALL 3. GROUND WATER SCENARIO 4. WATER LEVEL 5. GROUND WATER RESOURCES 6. GROUND WATER QUALITY 7. STATUS OF GROUND WATER DEVELOPMENT 8. GROUND WATER MANAGEMENT STRATEGY 9. GROUND WATER DEVELOPMENT 10. WATER CONSERVATION & ARTIFICIAL RECHARGE 11. RECOMMENDATIONS KARIMNAGAR DISTRICT AT A GLANCE 1. GENERAL FEATURES: i. Location : North Latitude 180 00' and 190 00' : East Longitude 780 40' and 800 00' ii. Geographical area : 11823 Sq kms iii. Dist head quarters : Karimnagar iv. No. of Revenue. Mandals : Fifty Seven (57) v. No. of Revenue. villages : One thousand fifty eight (1058). vi. Population (2011) : a) Total - 38, 11,738 b) Urban- 9,94, 231 c) Rural- 28,17,507 vii. Population density : 322/ sq.km 2. RAINFALL(2012 in mm): i. Normal Annual Rainfall : 1521.0 Monsoon Rainfall : 83% Non-monsoon Rainfall : 11% ii. Cumulative departure for : 32% to 73% the last 5 yrs from normal 3. LAND USE (2012) (Area in ha) i. Forest : 7,59,438 ii. Barren and uncultivable land : 88,887 iii. -

Jagtial District
JAGTIAL DISTRICT We acknowledge the content from http://jagitial.telangana.gov.in/district-profile Jagtial district is one of the 31 districts in the state of Telangana. Jagtial was carved out of Karimnagar district and was made as a district on October 11, 2016. Jagtial district is spread across an area of 3,043 square kilometers and has a population of 9,83,414 as per 2011 Census data. Jagtial town is the headquarters with a population of 1,03,962. Korutla is the second largest town with a population of 1,08,297. Jagtial and Metpally are the revenue divisions in this district. These are further divided into 18 mandals. Jagtial, Korutla, Dharmapuri, Choppadondi and Vemulawada Assembly constituencies are under Jagtial district. Jagtial town got its name from Jaggadeva. Godavari River flows through this district. There is Acharya N. G. Ranga Agricultural University's Agricultural College in Polasa. Kotilingala located on the bank of Godavari River was an important town in ancient town during the period of the Assaka mahajanapada and the Satavahanas. Lakshminarasimha Swamy Temple at Dharmapuri and Anjaneya Swamy Temple at Kondagattu are famous temples. There is sugar factory located at Muthyampeta. PHYSICAL FEATURES OF JAGTIAL DISTRICT: HISTORICAL PLACES: The historical Qila at Jagtial. TEMPLES: The holy temple of Sri Laxminarsimha Swamy on the banks of the Godavari River at Dharmapuri Mandal head-quarters The holy temple of Sri Koteshwara Swamy on the banks of the Godavari River at Kotilingala village in Velgatur Mandal The holy temple of Sri Anjaneya Swamy located at Kondagattu of Muthyampet village in Mallial Mandal. -

S.No Bank Mandal Village Population 1 State Bank of India
SLBC OF AP CONVENOR:ANDHRA BANK FIP - ABOVE 2000 POPULATION VILLAGES Name of the District : Karimnagar S.No Bank Mandal Village Population 1 State Bank of India KARIMNAGAR REKURTHI 5047 2 KARIMNAGAR MALKAPUR 2372 3 THIMMAPUR NEDUNOOR 2077 4 THIMMAPUR RENUKUNTA 2947 5 THIMMAPUR VACHUNOOR 2249 6 KARIMNAGAR NAGUNOOR 4664 7 KARIMNAGAR AREPALLI 3442 8 KAMANPUR KANNAL 6839 9 JAGTIAL CHELGAL 5037 10 JAGTIAL DHARUR 2513 11 JAGTIAL JABITHAPUR 3175 12 JAGTIAL MOTHE 4309 13 JAGTIAL NARSINGAPUR 4282 14 JAGTIAL THIPPENNAPET 3496 15 GOLLAPALLY MADDUNOOR 2745 16 DHARMAPURI VENUGUMETLA 2364 17 GOLLAPALLY BADNAKAL 2591 18 MUSTABAD CHIKODU 3449 19 MUSTABAD MALLAREDDIPET 3050 20 GAMBHIRRAOPET NAMAPURAM 3337 21 MUSTABAD THERLAMADDI 3758 22 Syndicate Bank MUSTABAD DHURSHED 2435 23 MALLAPUR CHITTAPUR 24 MALLAPUR SATHARAM 25 MALLAPUR DHARMARAJPALLI 26 State Bank of Hyderabad KARIMNAGAR YELLABHOTHARAM 2239 27 KARIMNAGAR CHINTAKUNTA 3123 28 MANAKONDUR KALLEDA 3688 29 MANAKONDUR PACHUNOOR 3839 30 MANAKONDUR VEGURPALLI 2123 31 THIMMAPUR MANNEMPALLI 2414 32 THIMMAPUR NALLAGONDA 2485 33 THIMMAPUR NUSTULLAPURAM 7411 34 GANGADHARA KURIKYALA 3027 35 GANGADHARA MALLAPUR 2314 36 GANGADHARA NARAYANAPURAM 2215 37 HUZURABAD BORNAPALLI 4621 38 HUZURABAD CHELPUR 7266 39 HUZURABAD JOOPAKA 2012 40 HUZURABAD PENCHIKALAPETA 3581 41 JAMMIKUNTA DHARMARAM (PB) 10030 42 JAMMIKUNTA ELLANTHAKUNTA 6008 43 JAMMIKUNTA KANAGARTHI 2216 44 JAMMIKUNTA MEDIPALLI 4385 45 BHEEMADEVARAPALLY BHEEMADEVARAPALL 2393 46 BHEEMADEVARAPALLY ERRAPALLII 2839 47 BHEEMADEVARAPALLY KOTHAPALLII 3883 48 BHEEMADEVARAPALLY -
The Andhra Pradesh Reorganisation Act 2014
jftLVªh lañ Mhñ ,yñ—(,u)04@0007@2003—14 REGISTERED NO. DL—(N)04/0007/2003—14 vlk/kkj.k EXTRAORDINARY Hkkx II — [k.M 1 PART II — Section 1 izkf/kdkj ls izdkf'kr PUBLISHED BY AUTHORITY lañ 6] ubZ fnYyh] 'kfuokj] ekpZ 1] 2014@ QkYxqu 10] 1935 ¼'kd½ No. 6] NEW DELHI, SATURDAY, MARCH 1, 2014/PHALGUNA 10, 1935 (SAKA) bl Hkkx esa fHkUu i`"B la[;k nh tkrh gS ftlls fd ;g vyx ladyu ds :i esa j[kk tk ldsA Separate paging is given to this Part in order that it may be filed as a separate compilation. MINISTRY OF LAW AND JUSTICE (Legislative Department) New Delhi, the 1st March, 2014/Phalguna 10, 1935 (Saka) The following Act of Parliament received the assent of the President on the 1st March, 2014, and is hereby published for general information:— THE ANDHRA PRADESH REORGANISATION ACT, 2014 NO. 6 OF 2014 [1st March, 2014.] An Act to provide for the reorganisation of the existing State of Andhra Pradesh and for matters connected therewith. BE it enacted by Parliament in the Sixty-fifth Year of the Republic of India as follows:— PART I PRELIMINARY 1. This Act may be called the Andhra Pradesh Reorganisation Act, 2014. Short title. 2. In this Act, unless the context otherwise requires,— Definitions. (a) “appointed day” means the day which the Central Government may, by notification in the Official Gazette, appoint; (b) “article” means an article of the Constitution; (c) “assembly constituency”, “council constituency” and “parliamentary constituency” have the same meanings as in the Representation of the People 43 of 1950. -
Peddapalle-Constituency Wise Brochure Final V13.Cdr
Passenger Amenities Station Amenity Amount Status Odela New Platform ` 30 lakh Constructed Provision of Passenger Amenities and Raghavapuram Circulating Area ` 29 lakh Developed facilities, and its improvements at Railway ` Stations is one of the most important aspect 4 Stations Wi-Fi Facility 6 lakh Provided of Railway Services. Mancherial Two Lifts ` 1 crore Provided Ramagundam Informative Mancherial Signages ` 2.10 crore Proposed Ramagundam 32 CCTV & Macheryal Cameras ` 74 lakh Proposed Mancherial Two ATVMs Ramagundam Two ATVMs ` 6 lakh Provided Mancherial CoTVM ` 4.5 lakh Provided Mancherial CIB/TIB Ramagudam CIB/TIB ` 2.06 crore Provided Mancherial Facade Improvement ` 28 lakh Completed Bellampalli PF Shelter ` 10 lakh Completed Shri Narendra Modi Ramagundam 2 Lifts ` 1 crore In Progress Hon’ble Prime Minister Mancherial Illumination Improved Ravindrakhani Platform Mandamarri construction/ ` 4.49 crore In Progress Ramagundam extension RAIL DEVELOPMENTAL WORKS Manchiryal Peddapalle Parliamentary Constituency Jurisdiction Passenger Train Services Details of Provision of New Trains/Stoppages/Capacity Enhancement for Trains in Peddapalle Parliamentary Constituency Jurisdiction. New Trains Introduced South Central Railway serving the entire state of 1. Tr.No.22415/22416 Visakhapatnam – New Delhi – Visakhapatnam Superfast AP Express Telangana, major portion Provision of Stoppages of Andhra Pradesh and 1. Tr.No.22128/22127 Kazipet – Lokamanya Tilak (T) – Kazipet Weekly superfast express has been given considerable portion of SOUTH CENTRAL stoppage at Peddapalli Railway station. Karnataka, Maharastra 2. Tr.No.11084/11083 Kazipet – Lokamanya Tilak (T) – Kazipet Weekly express has been given stoppage at states and small parts RAILWAY Peddapalli Railway station. of Tamilnadu and 3. Tr.No.22151/22152 Pune – Lokamanya Tilak (T) – Pune Weekly superfast express has been given stoppage Madhya Pradesh. -

Draft Electoral Roll of Medak-Nizamabad-Adilabad-Karimnagar Teachers Constituency of the A.P Legislative Council As Published on 15-12-2012
Draft Electoral Roll of Medak-Nizamabad-Adilabad-Karimnagar Teachers Constituency of the A.P Legislative Council as published on 15-12-2012 Polling Station Number : ( 158 ) Ibrahimpatnam District: Karimnagar - 20 Zilla Parishad Secendary School Ibrahimpatnam Sl.No. House address Full Name of the Name of father/ mother / Name of educational Age (Place of ordinary elector husband institution, if any, in residence) which he is teaching (1) (2) (3) (4) (5) (6) Mandal : IBRAHIMPATNAM Village: BANDALINGAPUR 2-1-123 Thammala Jagadishwar Bhumaiah Z.P.H.S., GODUR 40 1 BANDALINGAPUR 8-58 Bollu Shankar Bollu Ramulu Z.P.H.S.,RAJESHWARRAOP 33 2 SCHOOL ROAD ET 9-48 Eaga Sridhar Kumar Chakradhar Z.P.H.S., MEDIPALLI 39 3 SRI SATHYASAI STREET 9-63 Pandena Dasharatham Gangaram Z.P.H.S., SATTAKKAPALLI 39 4 MAIN ROAD BUS STOP 10-61 G Narshimha Chary Kishnama Chary Z.P.H.S.,ATHMAKUR 36 5 BANDALINGAPOOR Mandal : IBRAHIMPATNAM Village: ERRAPUR 2-34 Lavudya Skylab Poshetti Z.P.H.S., 26 6 ERRAPUR IBRAHIMPATNAM Mandal : IBRAHIMPATNAM Village: GODUR 1-49 Gurudu Suresh Pedda Gangaram Z.P.H.S., 39 7 GODHUR IBRAHIMPATNAM 1-49 Gurudu Ganesh Gangaram Z.P.H.S.,THIMMAPUR 46 8 GODUR 3-75 Podeti Narsaiah Bhoomaiah Z.P.H.S., 36 9 OLD S.C.COLONY IBRAHIMPATNAM Mandal : IBRAHIMPATNAM Village: IBRAHIMPATNAM 2-51 Naini Kalidas Naganna Z.P.H.S., SANGAMPALLY 49 10 VARSHAKONDA 3-14 Akula Sadanandam Ramaiah Z.P.H.S.,VARSHAKONDA 50 11 VARSHAKONDA 9-6 Jangili Ashok Somanna Z.P.H.S., GODUR 31 12 MUNNURU KAPU WADA 12-10 Margam Rajendhar Narayana Z.P.H.S.,VARSHAKONDA 29 13 IBRAHIMPATNAM 16-41/1 Syed Raheem Pasha Chand Z.P.H.S., VELLULLA 39 14 IBRAHIMPATNAM 18-32 Mattela Rathnakar Rajeshwer Z.P.H.S., JAGGASAGAR 39 15 IBRAHIMPATNAM 1of 374 Draft Electoral Roll of Medak-Nizamabad-Adilabad-Karimnagar Teachers Constituency of the A.P Legislative Council as published on 15-12-2012 Polling Station Number : ( 158 ) Ibrahimpatnam District: Karimnagar - 20 Zilla Parishad Secendary School Ibrahimpatnam Sl.No. -
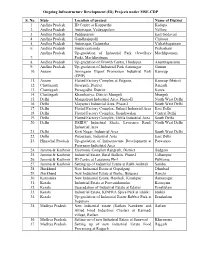
Ongoing Infrastructure Development (ID) Projects Under MSE-CDP
Ongoing Infrastructure Development (ID) Projects under MSE-CDP S. No. State Location of project Name of District 1. Andhra Pradesh ID Centre at Kopparthy Kadapa 2. Andhra Pradesh Autonagar, Vedayapalem Nellore 3. Andhra Pradesh Peddapuram East Godavari 4. Andhra Pradesh Gandhrajupalli Chittoor 5. Andhra Pradesh Autonagar, Gajuwaka Vishakhapatnam 6. Andhra Pradesh Singarayakonda Prakasham Andhra Pradesh Up-gradation of Industrial Park (Jewellery Machlipatnam 7. Park), Machlipatnam 8. Andhra Pradesh Up-gradation of Growth Centre, Hindupur Ananthapuramu 9. Andhra Pradesh Up-gradation of Industrial Park Autonagar Guntur 10. Assam Amingaon Export Promotion Industrial Park Kamrup (EPIP) 11. Assam Flatted Factory Complex at Patgaon, Kamrup (Metro) 12. Chattisgarh Siyarpali, District Raigarh 13. Chattisgarh Parasgadhi, District Korea 14. Chattisgarh Khamhariya, District Mungeli Mungeli 15. Delhi Mangolpuri Industrial Area, Phase-II North West Delhi 16. Delhi Mayapuri Industrial Area, Phase-I South West Delhi 17. Delhi Flatted Factory Complex, Jhilmil Industrial Area East Delhi 18. Delhi Flatted Factory Complex, Jhandewalan Central Delhi 19. Delhi Flatted Factory Complex, Okhla Industrial Area South Delhi 20. Delhi DSIIDC Industrial Sheds, Lawrence Road, North West Delhi Industrial Area 21. Delhi Kirti Nagar, Industrial Area South West Delhi 22. Delhi Patparganj, Industrial Area East Delhi 23. Himachal Pradesh Up-gradation of Infrastructure Development at Parwanoo Parwanoo Industrial Area 24. Jammu & Kashmir Electronic Complex Rangreth, District Badgam 25. Jammu & Kashmir Industrial Estate, Batal Ballain, Phase-I Udhampur 26. Jammu & Kashmir ID Centre at Lassipora Ph-I Pulwama 27. Jammu & Kashmir Setting up of Industrial Estate at Rakh Ambtali Samba 28. Jharkhand New Industrial Estate at Gopalganj Dhanbad 29. Jharkhand New Industrial Estate at Barhe, Bijupara Ranchi 30.