Impact of Y Chromosome Azfc Subdeletion Shows Lower Risk Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
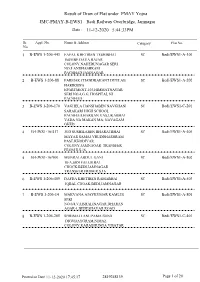
Jmc-Pmay-B-Ews1 11-12-2020 5:44:33Pm
Result of Draw of Flat under PMAY Yojna JMC-PMAY-B-EWS1 Bedi Railway Overbridge, Jamnagar Date : 11-12-2020 5:44:33PM Sr. Appl. No. Name & Address Category Flat No. No. 1 B-EWS 1-206-443 FAFAL KHETIBEN TESHIBHAI SC Bedi/EWS1-A-106 BOMBE DAVA BAJAR COLONY,NAHERUNAGAR SERI NO.5 ANDHASHRAM PACHHAD,JAMNAGAR 2 B-EWS 1-206-88 PARMAR CHANDRAKANT DHULAB SC Bedi/EWS1-A-202 HARIKRIPA EPARTMENT-103,HIMMATNAGAR SERI NO.4,G.G. HOSPITAL NI PACHHAD 3 B-EWS 1-206-170 VAGHELA HANSHABEN NAVGHANB SC Bedi/EWS1-C-201 SARAKARI HIGH SCHOOL PACHHAD,HARIJAN VAS,UKABHAI VADA NA MAKAN MA, NAVAGAM GHED 4 B-EWS1-16/417 JOD SUSHILABEN BHARATBHAI SC Bedi/EWS1-A-406 MAYAR SAMAJ,VRUDDHASHRAM PASE,KHODIYAR COLONY,JAMNAGAR TRANSFER FROM R.S.16 5 B-EWS1-16/900 MUNRAI ABDUL GANI SC Bedi/EWS1-A-802 18-A,SIDI FALI,IKBAL CHOCK,BEDI,JAMNAGAR TRANSFER FROM R.S.16 6 B-EWS 1-206-409 DAFDA KHETIBEN RAMABHAI SC Bedi/EWS1-A-503 IQBAL CHOAK,BEDI,JAMNAGAR 7 B-EWS 1-206-14 MAKVANA AJAYKUMAR KAMLES SC Bedi/EWS1-A-801 SERI NO.6/4,VAISHALINAGAR,DHARAN AGAR-1,BEDESHAVAR ROAD 8 B-EWS 1-206-205 SHRIMALI ANUPAMA SUNIL SC Bedi/EWS1-C-401 DIGVIJAYGRAM,NIMAZ COLONY,KARABHUNGA VISATAR Printed on Date 11-12-2020 17:45:17 2819248139 Page 1 of 20 Result of Draw of Flat under PMAY Yojna JMC-PMAY-B-EWS1 Bedi Railway Overbridge, Jamnagar Date : 11-12-2020 5:44:33PM Sr. -

Custodians of Culture and Biodiversity
Custodians of culture and biodiversity Indigenous peoples take charge of their challenges and opportunities Anita Kelles-Viitanen for IFAD Funded by the IFAD Innovation Mainstreaming Initiative and the Government of Finland The opinions expressed in this manual are those of the authors and do not nec - essarily represent those of IFAD. The designations employed and the presenta - tion of material in this publication do not imply the expression of any opinion whatsoever on the part of IFAD concerning the legal status of any country, terri - tory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The designations “developed” and “developing” countries are in - tended for statistical convenience and do not necessarily express a judgement about the stage reached in the development process by a particular country or area. This manual contains draft material that has not been subject to formal re - view. It is circulated for review and to stimulate discussion and critical comment. The text has not been edited. On the cover, a detail from a Chinese painting from collections of Anita Kelles-Viitanen CUSTODIANS OF CULTURE AND BIODIVERSITY Indigenous peoples take charge of their challenges and opportunities Anita Kelles-Viitanen For IFAD Funded by the IFAD Innovation Mainstreaming Initiative and the Government of Finland Table of Contents Executive summary 1 I Objective of the study 2 II Results with recommendations 2 1. Introduction 2 2. Poverty 3 3. Livelihoods 3 4. Global warming 4 5. Land 5 6. Biodiversity and natural resource management 6 7. Indigenous Culture 7 8. Gender 8 9. -

Ethnographic Series, Sidhi, Part IV-B, No-1, Vol-V
CENSUS OF INDIA 1961 VOLUMEV, PART IV-B, No.1 ETHNOGRAPHIC SERIES GUJARAT Preliminary R. M. V ANKANI, investigation Tabulation Officer, and draft: Office of the CensuS Superintendent, Gujarat. SID I Supplementary V. A. DHAGIA, A NEGROID L IBE investigation: Tabulation Officer, Office of the Census Superintendent, OF GU ARAT Gujarat. M. L. SAH, Jr. Investigator, Office of the Registrar General, India. Fieta guidance, N. G. NAG, supervision and Research Officer, revised draft: Office of the Registrar General, India. Editors: R. K. TRIVEDI, Su perintendent of Census Operations, Gujarat. B. K. Roy BURMAN, Officer on Special Duty, (Handicrafts and Social Studies), Office of the Registrar General, India. K. F. PATEL, R. K. TRIVEDI Deputy Superintendent of Census Superintendent of Census Operations, Gujarat. Operations, Gujarat N. G. NAG, Research Officer, Office' of the Registrar General, India. CENSUS OF INDIA 1961 LIST OF PUBLICATIONS CENTRAL GOVERNMENT PUBLICATIONS Census of India, 1961 Volume V-Gujarat is being published in the following parts: '" I-A(i) General Report '" I-A(ii)a " '" I-A(ii)b " '" I-A(iii) General Report-Economic Trends and Projections :« I-B Report on Vital Statistics and Fertility Survey :I' I-C Subsidiary Tables '" II-A General Population Tables '" II-B(I) General Economic Tables (Tables B-1 to B-IV-C) '" II-B(2) General Economic Tables (Tables B-V to B-IX) '" II-C Cultural and Migration Tables :t< III Household Economic Tables (Tables B-X to B-XVII) "'IV-A Report on Housing and Establishments :t<IV-B Housing and Establishment -

Analysis of Y-Chromosome Polymorphisms in Pakistani
ANALYSIS OF Y-CHROMOSOME POLYMORPHISMS IN PAKISTANI POPULATIONS Thesis submitted to the Sindh Institute of Medical Sciences for the degree of Doctor of Philosophy. BY Sadaf Firasat Centre of Human Genetics and Molecular Medicine Sindh Institute of Medical Sciences Sindh Institute of Urology and Transplantation (SIUT) Karachi, Pakistan 2010 TABLE OF CONTENTS Title page Acknowledgements ii List of Tables iii List of Figures iv Summary vi Introduction 1 Literature Review 19 Materials and Methods 34 Results Phylogeography of Pakistani ethnic groups. 51 Comparison between the Pakistani and Greek populations 73 Discussion 86 Comparison within Pakistan 88 Comparison between the Pakistani and Greek population 94 Comparison with world populations 98 Insight in to populations origins 111 Conclusions 121 References 122 Appendix a i ACKNOWLEDGEMENT I thank Prof. Dr. Syed Qasim Mehdi H.I. S.I., for his support, encouragement and for providing all the facilities for doing scientific work in his laboratory. The work presented in this thesis was done under the supervision of Dr. Qasim Ayub T.I. It is great pleasure for me to acknowledge the keen interest, advice, patient guidance and kindness that I have received from him during the course of this work. I would like to thank Dr. Shagufta Khaliq, (PoP), for teaching all the molecular genetics lab techniques and also to Dr Aiysha Abid for comments on this manuscript and suggestion for its improvement. I am also grateful to Mrs. Ambreen Ayub for her help in making the contour map. I thank my colleague Ms. Sadia Ajaz for her help and cooperation in proof reading the thesis. -

An African Indian Community in Hyderabad Siddi Identity, Its
An African Indian Community in Hyderabad Siddi Identity, Its Maintenance and Change Dissertation Zur Erlangung des sozialwissenschaftlichen Doktorgrades der Sozialwissenschaftlichen Fakultat der Georg-August-Universitat Gottingen vorgelegt von Ababu Minda Yimene aus Debre Birhan, Athiopien Gottingen 2004 Table of Contents Table of Contents i List of Maps v List of Tables v List of Figures v List of Illustrations vii Acknowledgements ix Preface xi 1. INTRODUCTION 1 LlProfile of the Study Area / 1.1.1 Hyderabad 1 1.1.2 A Glimpse on the History of Hyderabad 2 1.1.3 Geography and Climate 5 1.2 Objectives and Significance of the Study 10 1.3 Methodologies 13 1.3.1 Participant Observation 13 1.3.2 Interviews 13 1.3.3 Group Discussions 14 1.3.4 Literature Review 14 1.3.5 Biographies 14 1.3.6 Oral Traditions 15 1.3.7 Network Analysis 15 1.4 Informants and Interpreters 16 2. CONCEPTS OF ETHNICITY 19 2.1 Ethnic Group 21 2.2 Ethnicity 28 2.2.1 Primordialism 28 2.2.2 Instrumentalism 29 2.2.3 Combining Both Approaches 30 2.3 Migration and Ethnicity 32 2.4 Diaspora and Ethnicity 35 3. CONCEPTS OF SLAVERY AND SERVILE INSTITUTIONS ... .39 3.1 Slavery in Greek and Roman Antiquity 41 3.1.1 Aristotelian View of Slavery 42 3.1.2 Stoical View of Slavery 43 3.2 Medieval Slavery - The Ottoman Empire 45 3.3 African Slavery - The Case of Ethiopia 48 3.4 Modern Slavery - America 55 3.5 Cross-Cultural Notions of Slavery 57 4. -

Annotated Bibliography of Studies on Muslims in India
Studies on Muslims in India An Annotated Bibliography With Focus on Muslims in Andhra Pradesh (Volume: ) EMPLOYMENT AND RESERVATIONS FOR MUSLIMS By Dr.P.H.MOHAMMAD AND Dr. S. LAXMAN RAO Supervised by Dr.Masood Ali Khan and Dr.Mazher Hussain CONFEDERATION OF VOLUNTARY ASSOCIATIONS (COVA) Hyderabad (A. P.), India 2003 Index Foreword Preface Introduction Employment Status of Muslims: All India Level 1. Mushirul Hasan (2003) In Search of Integration and Identity – Indian Muslims Since Independence. Economic and Political Weekly (Special Number) Volume XXXVIII, Nos. 45, 46 and 47, November, 1988. 2. Saxena, N.C., “Public Employment and Educational Backwardness Among Muslims in India”, Man and Development, December 1983 (Vol. V, No 4). 3. “Employment: Statistics of Muslims under Central Government, 1981,” Muslim India, January, 1986 (Source: Gopal Singh Panel Report on Minorities, Vol. II). 4. “Government of India: Statistics Relating to Senior Officers up to Joint-Secretary Level,” Muslim India, November, 1992. 5. “Muslim Judges of High Courts (As on 01.01.1992),” Muslim India, July 1992. 6. “Government Scheme of Pre-Examination Coaching for Candidates for Various Examination/Courses,” Muslim India, February 1992. 7. National Sample Survey Organization (NSSO), Department of Statistics, Government of India, Employment and Unemployment Situation Among Religious Groups in India: 1993-94 (Fifth Quinquennial Survey, NSS 50th Round, July 1993-June 1994), Report No: 438, June 1998. 8. Employment and Unemployment Situation among Religious Groups in India 1999-2000. NSS 55th Round (July 1999-June 2000) Ministry of Statistics and Programme Implementation, Government of India, September 2001. Employment Status of Muslims in Andhra Pradesh 9. -

Jihadist Violence: the Indian Threat
JIHADIST VIOLENCE: THE INDIAN THREAT By Stephen Tankel Jihadist Violence: The Indian Threat 1 Available from : Asia Program Woodrow Wilson International Center for Scholars One Woodrow Wilson Plaza 1300 Pennsylvania Avenue NW Washington, DC 20004-3027 www.wilsoncenter.org/program/asia-program ISBN: 978-1-938027-34-5 THE WOODROW WILSON INTERNATIONAL CENTER FOR SCHOLARS, established by Congress in 1968 and headquartered in Washington, D.C., is a living national memorial to President Wilson. The Center’s mission is to commemorate the ideals and concerns of Woodrow Wilson by providing a link between the worlds of ideas and policy, while fostering research, study, discussion, and collaboration among a broad spectrum of individuals concerned with policy and scholarship in national and interna- tional affairs. Supported by public and private funds, the Center is a nonpartisan insti- tution engaged in the study of national and world affairs. It establishes and maintains a neutral forum for free, open, and informed dialogue. Conclusions or opinions expressed in Center publications and programs are those of the authors and speakers and do not necessarily reflect the views of the Center staff, fellows, trustees, advisory groups, or any individuals or organizations that provide financial support to the Center. The Center is the publisher of The Wilson Quarterly and home of Woodrow Wilson Center Press, dialogue radio and television. For more information about the Center’s activities and publications, please visit us on the web at www.wilsoncenter.org. BOARD OF TRUSTEES Thomas R. Nides, Chairman of the Board Sander R. Gerber, Vice Chairman Jane Harman, Director, President and CEO Public members: James H. -

Official~W1gazette
REGD.GOA-5 t Panaji, 2nd March, 2000 (Phalguna 12, 1921) \\t,/ SERIES I No. 49 . "'" OFFICIAL~W1GAZETTE. ~- -! - . GOVERNMENT OF GOA GOVERNMENT OF GOA 2. Amendment of' rule 2.- In rule 2 of the Goa Sales Tax Rules, 1964, in clause (e) after the existing Department of Finance proviso, the following proviso shall be inserted, namely.- Revenue and Expenditure Division "Provid"d further that the Sales Tax Officer/ Assis tant Sales Tax Officer posted in the office of the Commissioner of Sales Tax, Panaji, shall be the c Appropriate Assessing Authority in respect of such Notification dealers and for such purposes, including registrations, 5/13/95-Fin (R&C) assessment, re-assessment, recovery, enforcement. etc .. as the Commissioner or any other Officer authorised Whereas certain draft rules further to amend the Goa by him not below the rank of Assistant Commissioner Sales Tax Rules\ 1964, were published as reguired by of Sales Tax, may, by a special or general order, sub-section (I) of section 36.of the Goa Sales Tax Act, specify." - 1964 (Act 4 of 1964), in the Official Gazette, Series I No. 40, dated 30-12-1999, under Notification By order and in the name of the Governor of Goa. No. 5/13/95-Fin (R&C) dated 21-12-1999 of the 1. F. A. Rodrigues, Under Secretary (Fin. Exp.). Department of Finance, Revenue and Control Division, Panaji, ~3rd February, 2000. inviting objections and suggestions from all persons likely to be affected thereby, before the expiry of thirty days from the date of publication of the said Notification in Amendment the Official Gazette; 12/7/86-Fin(R&C) And whereas the said Gazette was made available Read:- Government Order No. -

Janjira Fort-Siddhi Architecture of India
Janjira Fort-Siddhi Architecture of India Dr Uday Dokras B.Sc., B.A. (Managerial Economics), LLB. Nagpur University,India Graduate Studies,Queen’s University, Canada MBA (CALSTATE,USA) Graduate Diploma in Law, Stockholm University,Sweden Ph.D (Management) Stockholm University, Sweden CONSULTANT- Gorewada International Zoo, Nagpur,India- Largest Zoo and Safari in Asia Srishti Dokras B.Arch. (Institute for Design Education and Architectural Studies) Nagpur India Visiting Architect, Australia & USA Consultant - Design and Architecture, Esselworld Gorewada International Zoo 1 A B S T R A C T Janjira - The Undefeated Fort Janjira Fort is situated on the Murud beach in the Arabian sea along the Konkan coast line. Murud is the nearest town to the fort which is located at about 165 kms from Mumbai. You need to drive on the NH17 till Pen & then proceed towards Murud via Alibaug and Revdanda. The Rajapuri jetty is from where sail boats sail to the fort entrance. The road from murud town to janjira fort takes you a top a small hill from where you get the first glimpse of this amazing fort. Once you decent this hill, you reach Rajapuri jetty which is a small fishermen village. The sail boats take you from the jetty to the main door of the fort . One unique feature of this fort is that the entrance is not easily visible from a distance and can only be identified, once you go nearer to the walls of the fort. This was a strategy due to which Janjira was never conquered as the enemy would just keep on wondering about the entrance of the fort. -

2021 Daily Prayer Guide for All People Groups & LR-Unreached People Groups = LR-Upgs
2021 Daily Prayer Guide for all People Groups & LR-Unreached People Groups = LR-UPGs - of INDIA Source: Joshua Project data, www.joshuaproject.net Western edition To order prayer resources or for inquiries, contact email: [email protected] I give credit & thanks to Create International for permission to use their PG photos. 2021 Daily Prayer Guide for all People Groups & LR-UPGs = Least-Reached-Unreached People Groups of India INDIA SUMMARY: 2,717 total People Groups; 2,445 LR-UPG India has 1/3 of all UPGs in the world; the most of any country LR-UPG definition: 2% or less Evangelical & 5% or less Christian Frontier (FR) definition: 0% to 0.1% Christian Why pray--God loves lost: world UPGs = 7,407; Frontier = 5,042. Color code: green = begin new area; blue = begin new country Downloaded from www.joshuaproject.net in September 2020 * * * "Prayer is not the only thing we can can do, but it is the most important thing we can do!" * * * India ISO codes are used for some Indian states as follows: AN = Andeman & Nicobar. JH = Jharkhand OD = Odisha AP = Andhra Pradesh+Telangana JK = Jammu & Kashmir PB = Punjab AR = Arunachal Pradesh KA = Karnataka RJ = Rajasthan AS = Assam KL = Kerala SK = Sikkim BR = Bihar ML = Meghalaya TN = Tamil Nadu CT = Chhattisgarh MH = Maharashtra TR = Tripura DL = Delhi MN = Manipur UT = Uttarakhand GJ = Gujarat MP = Madhya Pradesh UP = Uttar Pradesh HP = Himachal Pradesh MZ = Mizoram WB = West Bengal HR = Haryana NL = Nagaland Why Should We Pray For Unreached People Groups? * Missions & salvation of all people is God's plan, God's will, God's heart, God's dream, Gen. -

21St123cindiasiddi.Pdf
UNITED NATIONS NATIONS UNIES 21st Century Producer: Mary Ferreira Script version: FINAL Duration: 8.36 THE SIDDIS: INDIA’S FORGOTTEN AFRICANS INTRO: Descended from African slaves – the Siddi people today live in western India. For centuries they’ve held on to their African culture – and a special relationship with the Asiatic lion. VIDEO AUDIO MONTAGE OF (MUSIC) FACES/DANCERS CULTURAL DANCE NARRATION FIRE They dance to the beat of the African drum, deep in the forest, mimicking a disappearing species that they’ve grown to love – the Asiatic lion. (10”) SIDDIS DANCING They’re a part of the 20,000 Siddis an ethnic group of African descent, who live in Gujarat, western India, (8”) DANCE TROUPE Though far removed from their ancestral lands, the Siddis have cherished their culture… now it’s a source of income for them. (15”) 1 IMRAN: (In Hindi) M IMRAN ON-CAM “We play in hotels for about 1,500 rupees or 25 US SOUNDBITE IN UNIA VERSION* dollars. We go to the hotel to dance for the tourists during the prime season.” (12”) NARRATION IMRAN PUTTING ON Imran is a Siddi, a descendant of the Bantu people MAKE UP from southeast Africa. (5”) He believes his ancestors originally came from Uganda. Now he lives in a small village called VILLAGE Jambur with his mother and grandmother. The FOREST village is surrounded by the forest of Gir, the last bastion of the world’s 500 remaining Asiatic lions. (18”) GRAPHIC & PHOTOS Centuries ago, Africans from Ethiopia, Eritrea and DOCUMENTING THE Somalia sailed to the Indian subcontinent as SLAVE TRADE - INDIA merchants - while others were brought as slaves. -

A Critical Semiotic Analysis of Language Textbooks
Education and Linguistics Research ISSN 2377-1356 2019, Vol. 5, No. 1 Power Relation Reproduction Through Images: A Critical Semiotic Analysis of Language Textbooks Hajra Yousif Pardesi English language development centre, Mehran University of Engineering and Technology, Jamshoro, Pakistan E-mail: [email protected] Received: April 5, 2019 Accepted: May 9, 2018 Published: May 17, 2019 doi:10.5296/elr.v5i1.14803 URL: https://doi.org/10.5296/elr.v5i1.14803 Abstract Images are in the form of pictures are often used to make books more attractive or more reader-friendly. Images are iconic signs that also convey some meaning. Unlike texts, the messages conveyed by images are less direct and affect human consciousness. This study offers critical analysis of images used in language textbooks from grade 1 to 5 used in government schools of Sindh, Pakistan. The aim of the study is to analyze what and how power relations are maintained and propagated through iconic signs, and how socioeconomic division and nationalist ideology is reproduced by the state through images. The analysis of images shows the portraying of reality in textbooks that legitimizes power relations. Based on Barthe’s image theory, the theoretical framework is adapted from Fitsumbirhan (2006) for the critical analysis of the images. Keywords: Power relation, Ideology, images, Hegemony, Nationalization, Visual representations, Mystification, Semiotic analysis 1. Introduction Power is the ability to influence or control the behavior of people. Power cannot be reduced to institutions and a group. Foucault (1980) maintains that, “power is everywhere, not because it embraces everything, but because it comes from everywhere.