Three-Dimensional Reconstruction of a Whole Insect Reveals Its Phloem Sap
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endoskeleton
THE EVOLUTION OF THE VERTEBRATE ENDOSKELETON AN ESSAY ON THE SIGNIFICANCE AND) MEANING OF' SEGMENTATION IN COELOMATE ANIMIALS By W1T. 13. PRIMIROSE, M.B.. Cii.B. Lately Seniior Deniionistrator of lAnatomtiy i? the (nWizversity (of Glasgozc THE EVOLUTION OF THE VERTEBRATE ENDOSKELETON WVHEN investigating the morphology of the vertebrate head, I found it necessary to discover the morphological principles on which the segmentation of the body is founded. This essay is one of the results of this investigations, and its object is to show what has determined the segmented form in vertebrate animals. It will be seen that the segmented form in vertebrates results from a condition which at no time occurs in vertebrate animals. This condition is a form of skeleton found only in animals lower in the scale of organisation than vertebrates, and has the characters of a space containing water. This space is the Coelomic Cavity. The coelomic cavity is the key to the formation of the segmented structure of the body, and is the structure that determines the vertebrate forni. The coclomic cavity is present in a well defined state from the Alnelida tuwards, so that in ainielides it is performing the functions for which a coelom was evolved. It is, however, necessary to observe the conditions prevailing anon)g still lower forms to see why a separate cavity was formed in animals, which became the means of raising them in the scale of organisation, and ultimately leading to the evolution of the vertebrate animal. I therefore propose to trace the steps in evolution by which, I presume, the coelomic cavity originated, and then show how it or its modifications have been the basis on which the whole vertebrate structure of animals is founded. -

Morphology and Adaptation of Immature Stages of Hemipteran Insects
© 2019 JETIR January 2019, Volume 6, Issue 1 www.jetir.org (ISSN-2349-5162) Morphology and Adaptation of Immature Stages of Hemipteran Insects Devina Seram and Yendrembam K Devi Assistant Professor, School of Agriculture, Lovely Professional University, Phagwara, Punjab Introduction Insect Adaptations An adaptation is an environmental change so an insect can better fit in and have a better chance of living. Insects are modified in many ways according to their environment. Insects can have adapted legs, mouthparts, body shapes, etc. which makes them easier to survive in the environment that they live in and these adaptations also help them get away from predators and other natural enemies. Here are some adaptations in the immature stages of important families of Hemiptera. Hemiptera are hemimetabolous exopterygotes with only egg and nymphal immature stages and are divided into two sub-orders, homoptera and heteroptera. The immature stages of homopteran families include Delphacidae, Fulgoridae, Cercopidae, Cicadidae, Membracidae, Cicadellidae, Psyllidae, Aleyrodidae, Aphididae, Phylloxeridae, Coccidae, Pseudococcidae, Diaspididae and heteropteran families Notonectidae, Corixidae, Belastomatidae, Nepidae, Hydrometridae, Gerridae, Veliidae, Cimicidae, Reduviidae, Pentatomidae, Lygaeidae, Coreidae, Tingitidae, Miridae will be discussed. Homopteran families 1. Delphacidae – Eg. plant hoppers They comprise the largest family of plant hoppers and are characterized by the presence of large, flattened spurs at the apex of their hind tibiae. Eggs are deposited inside plant tissues, elliptical in shape, colourless to whitish. Nymphs are similar in appearance to adults except for size, colour, under- developed wing pads and genitalia. 2. Fulgoridae – Eg. lantern bugs They can be recognized with their antennae inserted on the sides & beneath the eyes. -

First Record of the Aphid Genus Coloradoa Wilson (Hemiptera: Aphididae)
580 SHORT COMMUNICATIONS significant pathological changes in the uterine Pakistan J. Zool., vol. 47(2), pp. 580-585, 2015. endometrium that could hinder the embryonic implantation and further lead to infertility (Makker First Record of the Aphid Genus and Goel, 2013). Coloradoa Wilson (Hemiptera: This case study documents an interesting report which explains the incidence of uterine tumor Aphididae) from Saudi Arabia, with in buffaloes that could also act as a risk factor for some Morphological Notes on infertility. This case study would be informative for Variation in C. rufomaculata (Wilson, large animal clinicians and other veterinary related 1908) areas. Sabir Hussain, Yousif Aldryhim and Hathal Al- Conflict of interest Dhafer The authors have no conflict of interest. King Saud University Museum of Arthropods (KSMA), Plant Protection Department, College of References Food and Agriculture Sciences, King Saud Ashraf, S., Omer, A., Ijaz, M., Chaudry, U.N. and Ali, M.M., 2009. Pakistan J. Zool., 9 (Suppl):119-122. University, Riyadh, Kingdom of Saudi Arabia Avci, H., Serin, G., Aydoğan, A. and Birincioğlu, S., 2010. Turk. J. Vet. Anim. Sci., 34: 307-311. Abstract.- The aphid genus Coloradoa Wilson, is reported for the first time from the Azawi, O.I. and Al-Sadi, H.I., 2010. Uterine leiomyoma in a Kingdom of Saudi Arabia. The species buffalo: a case report. Buffalo Bull., 29: 80-82. Coloradoa rufomaculata (Wilson, 1908) was Baba, A.I. and Catoi, C., 2007. Comparative oncology. found feeding on Ambrosia maritima L. Romanian Academy, Bucharest. (Asteraceae) representing a new host plant. This Cooper, B.J. and Valentine, B.A., 2002. -

Insetos Do Brasil
COSTA LIMA INSETOS DO BRASIL 2.º TOMO HEMÍPTEROS ESCOLA NACIONAL DE AGRONOMIA SÉRIE DIDÁTICA N.º 3 - 1940 INSETOS DO BRASIL 2.º TOMO HEMÍPTEROS A. DA COSTA LIMA Professor Catedrático de Entomologia Agrícola da Escola Nacional de Agronomia Ex-Chefe de Laboratório do Instituto Oswaldo Cruz INSETOS DO BRASIL 2.º TOMO CAPÍTULO XXII HEMÍPTEROS ESCOLA NACIONAL DE AGRONOMIA SÉRIE DIDÁTICA N.º 3 - 1940 CONTEUDO CAPÍTULO XXII PÁGINA Ordem HEMÍPTERA ................................................................................................................................................ 3 Superfamília SCUTELLEROIDEA ............................................................................................................ 42 Superfamília COREOIDEA ............................................................................................................................... 79 Super família LYGAEOIDEA ................................................................................................................................. 97 Superfamília THAUMASTOTHERIOIDEA ............................................................................................... 124 Superfamília ARADOIDEA ................................................................................................................................... 125 Superfamília TINGITOIDEA .................................................................................................................................... 132 Superfamília REDUVIOIDEA ........................................................................................................................... -

New Insights Into the Microbiota of Moth Pests
International Journal of Molecular Sciences Review New Insights into the Microbiota of Moth Pests Valeria Mereghetti, Bessem Chouaia and Matteo Montagna * ID Dipartimento di Scienze Agrarie e Ambientali, Università degli Studi di Milano, 20122 Milan, Italy; [email protected] (V.M.); [email protected] (B.C.) * Correspondence: [email protected]; Tel.: +39-02-5031-6782 Received: 31 August 2017; Accepted: 14 November 2017; Published: 18 November 2017 Abstract: In recent years, next generation sequencing (NGS) technologies have helped to improve our understanding of the bacterial communities associated with insects, shedding light on their wide taxonomic and functional diversity. To date, little is known about the microbiota of lepidopterans, which includes some of the most damaging agricultural and forest pests worldwide. Studying their microbiota could help us better understand their ecology and offer insights into developing new pest control strategies. In this paper, we review the literature pertaining to the microbiota of lepidopterans with a focus on pests, and highlight potential recurrent patterns regarding microbiota structure and composition. Keywords: symbiosis; bacterial communities; crop pests; forest pests; Lepidoptera; next generation sequencing (NGS) technologies; diet; developmental stages 1. Introduction Insects represent the most successful taxa of eukaryotic life, being able to colonize almost all environments, including Antarctica, which is populated by some species of chironomids (e.g., Belgica antarctica, Eretmoptera murphyi, and Parochlus steinenii)[1,2]. Many insects are beneficial to plants, playing important roles in seed dispersal, pollination, and plant defense (by feeding upon herbivores, for example) [3]. On the other hand, there are also damaging insects that feed on crops, forest and ornamental plants, or stored products, and, for these reasons, are they considered pests. -

Faune De France Hémiptères Coreoidea Euro-Méditerranéens
1 FÉDÉRATION FRANÇAISE DES SOCIÉTÉS DE SCIENCES NATURELLES 57, rue Cuvier, 75232 Paris Cedex 05 FAUNE DE FRANCE FRANCE ET RÉGIONS LIMITROPHES 81 HÉMIPTÈRES COREOIDEA EUROMÉDITERRANÉENS Addenda et Corrigenda à apporter à l’ouvrage par Pierre MOULET Illustré de 3 planches de figures et d'une photographie couleur 2013 2 Addenda et Corrigenda à apporter à l’ouvrage « Hémiptères Coreoidea euro-méditerranéens » (Faune de France, vol. 81, 1995) Pierre MOULET Museum Requien, 67 rue Joseph Vernet, F – 84000 Avignon [email protected] Leptoglossus occidentalis Heidemann, 1910 (France) Photo J.-C. STREITO 3 Depuis la parution du volume Coreoidea de la série « Faune de France », de nombreuses publications, essentiellement faunistiques, ont paru qui permettent de préciser les données bio-écologiques ou la distribution de nombreuses espèces. Parmi ces publications il convient de signaler la « Checklist » de FARACI & RIZZOTTI-VLACH (1995) pour l’Italie, celle de V. PUTSHKOV & P. PUTSHKOV (1997) pour l’Ukraine, la seconde édition du « Verzeichnis der Wanzen Mitteleuropas » par GÜNTHER & SCHUSTER (2000) et l’impressionnante contribution de DOLLING (2006) dans le « Catalogue of the Heteroptera of the Palaearctic Region ». En outre, certains travaux qui m’avaient échappé ou m’étaient inconnus lors de la préparation de cet ouvrage ont été depuis ré-analysés ou étudiés. Enfin, les remarques qui m’ont été faites directement ou via des notes scientifiques sont ici discutées ; MATOCQ (1996) a fait paraître une longue série de corrections à laquelle on se reportera avec profit. - - - Glandes thoraciques : p. 10 ─ Ligne 10, après « considérés ici » ajouter la note infrapaginale suivante : Toutefois, DAVIDOVA-VILIMOVA, NEJEDLA & SCHAEFER (2000) ont observé une aire d’évaporation chez Corizus hyoscyami, Liorhyssus hyalinus, Brachycarenus tigrinus, Rhopalus maculatus et Rh. -

Tri-Ology Vol 58, No. 1
FDACS-P-00124 April - June 2020 Volume 59, Number 2 TRI- OLOGY A PUBLICATION FROM THE DIVISION OF PLANT INDUSTRY, BUREAU OF ENTOMOLOGY, NEMATOLOGY, AND PLANT PATHOLOGY Division Director, Trevor R. Smith, Ph.D. BOTANY ENTOMOLOGY NEMATOLOGY PLANT PATHOLOGY Providing information about plants: Identifying arthropods, taxonomic Providing certification programs and Offering plant disease diagnoses native, exotic, protected and weedy research and curating collections diagnoses of plant problems and information Florida Department of Agriculture and Consumer Services • Division of Plant Industry 1 Phaenomerus foveipennis (Morimoto), a conoderine weevil. Photo by Kyle E. Schnepp, DPI ABOUT TRI-OLOGY TABLE OF CONTENTS The Florida Department of Agriculture and Consumer Services- Division of Plant Industry’s (FDACS-DPI) Bureau of Entomology, HIGHLIGHTS 03 Nematology, and Plant Pathology (ENPP), including the Botany Noteworthy examples from the diagnostic groups Section, produces TRI-OLOGY four times a year, covering three throughout the ENPP Bureau. months of activity in each issue. The report includes detection activities from nursery plant inspections, routine and emergency program surveys, and BOTANY 04 requests for identification of plants and pests from the public. Samples are also occasionally sent from other states or countries Quarterly activity reports from Botany and selected plant identification samples. for identification or diagnosis. HOW TO CITE TRI-OLOGY Section Editor. Year. Section Name. P.J. Anderson and G.S. Hodges ENTOMOLOGY 07 (Editors). TRI-OLOGY Volume (number): page. [Date you accessed site.] Quarterly activity reports from Entomology and samples reported as new introductions or interceptions. For example: S.E. Halbert. 2015. Entomology Section. P.J. Anderson and G.S. -

Hemiptera: Heteroptera) of Magallanes Region: Checklist and Identification Key to the Species
Anales Instituto Patagonia (Chile), 2016. Vol. 44(1):39-42 39 The Coreoidea Leach, 1815 (Hemiptera: Heteroptera) of Magallanes Region: Checklist and identification key to the species Los Coreoidea Leach, 1815 (Hemiptera: Heteroptera) de la Región de Magallanes: Lista de especies y clave de identificación Eduardo I. Faúndez1,2 Abstract Slater, 1995), and several species are economically Members of the Coreoidea of Magallanes Region important; there are, however, also cases in which are listed. First records in the Magallanes Region are species of this superfamily have been recorded provided for Harmostes (Neoharmostes) procerus feeding on carrion and dung (Mitchell, 2000). Berg, 1878 and Althos nigropunctatus (Signoret, Additionally, biting humans has been recorded 1864). It is concluded that three species classified in members of this group (Faúndez & Carvajal, in three genera and two families are present in the 2011). In Chile, the Coreoidea is represented by region. A key to the species is provided. two families, the Coreidae and Rhopalidae, and the major diversity for this group is found in the central Key words: Coreidae, Rhopalidae, Distribution, zone of the country (Faúndez, 2015b). New records, Chile. In Magallanes, very little is known about the species of this superfamily, and actually there is Resumen only one species officially recorded from the area: Se listan los Coreoidea de la Region de Magallanes. the dunes bug, Eldarca nigroscutellata Faúndez, Se entregan los primeros registros para la región 2015 (Coreidae). The purpose of this contribution de Harmostes (Neoharmostes) procerus Berg, is to provide an update of this group in the 1878 y Althos nigropunctatus (Signoret, 1864). -

Large-Scale Gene Expression Reveals Different Adaptations of Hyalopterus Persikonus to Winter and Summer Host Plants
Insect Science (2017) 24, 431–442, DOI 10.1111/1744-7917.12336 ORIGINAL ARTICLE Large-scale gene expression reveals different adaptations of Hyalopterus persikonus to winter and summer host plants Na Cui1,4,∗, Peng-Cheng Yang2,∗, Kun Guo1,3, Le Kang1,4 and Feng Cui1 1State Key Laboratory of Integrated Management of Pest Insects & Rodents, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101; 2Beijing Institutes of Life Science, Chinese Academy of Sciences, Beijing 100101; 3Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing 100193, and 4University of Chinese Academy of Sciences, Beijing 100049, China Abstract Host alternation, an obligatory seasonal shifting between host plants of distant genetic relationship, has had significant consequences for the diversification and success of the superfamily of aphids. However, the underlying molecular mechanism remains unclear. In this study, the molecular mechanism of host alternation was explored through a large-scale gene expression analysis of the mealy aphid Hyalopterus persikonus on winter and summer host plants. More than four times as many unigenes of the mealy aphid were significantly upregulated on summer host Phragmites australis than on winter host Rosaceae plants. In order to identify gene candidates related to host alternation, the differentially expressed unigenes of H. persikonus were compared to salivary gland expressed genes and secretome of Acyrthosiphon pisum. Genes involved in ribosome and oxidative phosphorylation and with molecular functions of heme–copper terminal oxidase activity, hydrolase activity and ribosome binding were potentially upregulated in salivary glands of H. persikonus on the summer host. Putative secretory proteins, such as detoxification enzymes (carboxylesterases and cytochrome P450s), antioxidant enzymes (peroxidase and superoxide dismutase), glutathione peroxidase, glucose dehydrogenase, angiotensin-converting enzyme, cadherin, and calreticulin, were highly expressed in H. -

A Review of the Systematics of Hawaiian Planthoppers (Hemiptera: Fulgoroidea)L
Pacific Science (1997), vol. 51, no. 4: 366-376 © 1997 by University of Hawai'i Press. All rights reserved A Review of the Systematics of Hawaiian Planthoppers (Hemiptera: Fulgoroidea)l MANFRED ASCHE2 ABSTRACT: With 206 endemic species, the phytophagous Fulgoroidea, or planthop pers, are among the most important elements of the native Hawaiian fauna. These principally monophagous or oligophagous insects occur in nearly all Hawaiian terrestrial ecosystems. Species of two of the 18 planthopper families occurring worldwide have successfully colonized and subsequently radiated in Hawai'i. Based on collections made mainly by Perkins, Kirkaldy, Muir, Giffard, and Swezey, more than 95% of these species were described in the first three decades of this century. The systematics of the Hawaiian planthoppers has changed little in the past 60 yr and is not based on any phylogenetic analyses. This paper attempts a preliminary phylogenetic evaluation ofthe native Hawaiian p1anthoppers on the basis ofcompara tive morphology to recognize monophyletic taxa and major evolutionary lines. The following taxa are each descendants of single colonizing species: in Cixiidae, the Hawaiian Oliarus and Iolania species; in De1phacidae, Aloha partim, Dictyophoro delphax, Emoloana, Leialoha + Nesothoe, Nesodryas, and at least four groups within Nesosydne. Polyphyletic taxa are the tribe "Alohini," Aloha s.l., Nesorestias, Nesosydne s.l., and Nothorestias. Non-Hawaiian species currently placed in Iolania, Oliarus, Aloha, Leialoha, and Nesosydne are not closely allied to the Hawaiian taxa. The origin of the Hawaiian planthoppers is obscure. The Hawaiian Oliorus appear to have affinities to (North) American taxa. ALTHOUGH THE HAWAIIAN ISLANDS are the most Other groups of Hawaiian insects have isolated islands on earth, they house a remark received far less attention, although they are ably rich flora and fauna. -

Influence of Plant Parameters on Occurrence and Abundance Of
HORTICULTURAL ENTOMOLOGY Influence of Plant Parameters on Occurrence and Abundance of Arthropods in Residential Turfgrass 1 S. V. JOSEPH AND S. K. BRAMAN Department of Entomology, College of Agricultural and Environmental Sciences, University of Georgia, 1109 Experiment Street, GrifÞn, GA 30223-1797 J. Econ. Entomol. 102(3): 1116Ð1122 (2009) ABSTRACT The effect of taxa [common Bermuda grass, Cynodon dactylon (L.); centipedegrass, Eremochloa ophiuroides Munro Hack; St. Augustinegrass, Stenotaphrum secundatum [Walt.] Kuntze; and zoysiagrass, Zoysia spp.], density, height, and weed density on abundance of natural enemies, and their potential prey were evaluated in residential turf. Total predatory Heteroptera were most abundant in St. Augustinegrass and zoysiagrass and included Anthocoridae, Lasiochilidae, Geocoridae, and Miridae. Anthocoridae and Lasiochilidae were most common in St. Augustinegrass, and their abundance correlated positively with species of Blissidae and Delphacidae. Chinch bugs were present in all turf taxa, but were 23Ð47 times more abundant in St. Augustinegrass. Anthocorids/lasiochilids were more numerous on taller grasses, as were Blissidae, Delphacidae, Cicadellidae, and Cercopidae. Geocoridae and Miridae were most common in zoysiagrass and were collected in higher numbers with increasing weed density. However, no predatory Heteroptera were affected by grass density. Other beneÞcial insects such as staphylinids and parasitic Hymenoptera were captured most often in St. Augustinegrass and zoysiagrass. These differences in abundance could be in response to primary or alternate prey, or reßect the inßuence of turf microenvironmental characteristics. In this study, SimpsonÕs diversity index for predatory Heteroptera showed the greatest diversity and evenness in centipedegrass, whereas the herbivores and detritivores were most diverse in St. Augustinegrass lawns. These results demonstrate the complex role of plant taxa in structuring arthropod communities in turf. -
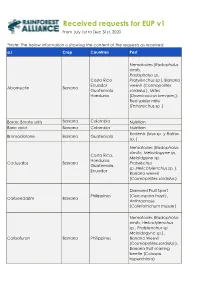
IIS 2020 Requests Overview
Received requests for EUP v1 From July 1st to Dec 31st, 2020 *Note: The below information is showing the content of the requests as received. a.i Crop Countries Pest Nematodes ( Radophulus similis, Radopholus sp, Costa Rica Pratylenchus sp ), Banana Ecuador weevil ( Cosmopolites Abamectin Banana Guatemala sordidus ), Mites Honduras (Dysmicoccus brevipes ), Red spider mite (Tetranychus sp .) Borax; Borate salts Banana Colombia Nutrition Boric acid Banana Colombia Nutrition Rodents ( Mus sp. y Rattus Bromadiolone Banana Guatemala sp. ) Nematodes ( Radopholus silmillis, Meloidogyne sp, Costa Rica, Meloidgune sp, Honduras, Cadusafos Banana Pratylechus Guatemala, sp.,Helicotylenchus sp. ), Ecuador Banana weevil (Cosmopolites sordidus ) Diamond Fruit Sport Philippines (Cercospora hayii ), Carbendazim Banana Anthracnose (Colletotrichum musae ) Nematod es (Radopholus similis, Helicotylenchus sp., Pratylenchus sp. Meloidogyne sp.) , Carbofuran Banana Philippines Banana Weevil (Cosmopolites sordidus ), Banana fruit scarring beetle (Colaspis hyperchlora) a.i Crop Countries Pest Black Sigatoka Ecuador, Costa (Mycosphaerella fijiensis ), Rica, Philippines, Yellow Sigatoka Chlorothalonil Banana Honduras, (Mycosphaerella Guatemala, musicola ), Banana Colombia Freckle ( Phyllosticta musarum ) Mealybug ( Dysmicoccus brevipes, Pseudococcus elisae, Pseudococcus sp, Ferrista sp ), Dysmicoccus sp , Scale insects (Aspidiotus destructor, Diaspis boisduvalii ), Thrips (Thrips florum, Franckliniella spp, Chaetanaphothrips signipennis ), Banana fruit Philippines,