Salamanders and Newts) Into the EU
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Zoology SYLLABUS 2016
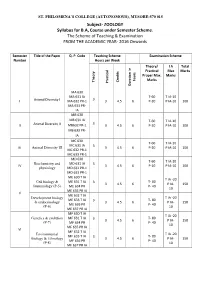
ST. PHILOMENA’S COLLEGE (AUTONOMOUS), MYSORE-570 015 Subject- ZOOLOGY Syllabus for B.A, Course under Semester Scheme. The Scheme of Teaching & Examination FROM THE ACADEMIC YEAR- 2016 Onwards Semester Title of the Paper Q. P. Code Teaching Scheme Examination Scheme Number Hours per Week Theory/ I A Total Practical Max Marks Proper Max. Marks hours Marks Theory Credits Practical Duration in in Duration MA 630 MA 631 IA T-60 T IA-10 3 I Animal Diversity I MA 632 PR-1 3 4.5 6 P-20 P IA-10 100 MA 633 PR- IA MB 630 MB 631 IA T-60 T IA-10 3 II Animal Diversity II MB632 PR-1 3 4.5 6 P-20 P IA-10 100 MB 633 PR- IA MC 630 T-60 T IA-10 MC 631 IA 3 III Animal Diversity III 3 4.5 6 P-20 P IA-10 100 MC 632 PR-1 MC 633 PR-1 MD 630 T-60 T IA-10 Biochemistry and MD 631 IA 3 IV 3 4.5 6 P-20 P IA-10 100 physiology MD 632 PR-1 MD 633 PR-1 ME 630 T IA T IA -20 Cell biology & ME 631 T IA 3 T- 80 3 4.5 6 P IA- 150 Immunology (P-5) ME 634 PR P- 40 10 ME 635 PR IA V ME 632 T IA Development biology T IA -20 ME 633 T IA 3 T- 80 & endocrinology 3 4.5 6 P IA- 150 ME 636 PR P- 40 (P-6) 10 ME 637 PR IA MF 630 T IA T IA -20 Genetics & evolution MF 631 T IA 3 T- 80 3 4.5 6 P IA- 150 (P-7) MF 634 PR P- 40 10 MF 635 PR IA VI MF 632 T IA Environmental T IA -20 MF 633 T IA 3 T- 80 Biology & Ethnology 3 4.5 6 P IA- 150 MF 636 PR P- 40 (P-8) 10 MF 637 PR IA ST. -

What Really Hampers Taxonomy and Conservation? a Riposte to Garnett and Christidis (2017)
Zootaxa 4317 (1): 179–184 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2017 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4317.1.10 http://zoobank.org/urn:lsid:zoobank.org:pub:88FA0944-D3CF-4A7D-B8FB-BAA6A3A76744 What really hampers taxonomy and conservation? A riposte to Garnett and Christidis (2017) MARCOS A. RAPOSO1,2, RENATA STOPIGLIA3,4, GUILHERME RENZO R. BRITO1,5, FLÁVIO A. BOCKMANN3,6, GUY M. KIRWAN1,7, JEAN GAYON2 & ALAIN DUBOIS4 1 Setor de Ornitologia, Departamento de Vertebrados, Museu Nacional, Universidade Federal do Rio de Janeiro, Quinta da Boa Vista, s/n, 20940–040 Rio de Janeiro, RJ, Brazil. [email protected] (MAR), [email protected] (GRRB), [email protected] (GMK) 2 UMR 8590, IHPST–Institut d'Histoire et de Philosophie des Sciences et des Techniques, UMR 8590, Université Paris 1 Panthéon- Sorbonne & CNRS, 13 rue du Four, 75006 Paris, France. [email protected] (JG) 3 Laboratório de Ictiologia de Ribeirão Preto, Departamento de Biologia, FFCLRP, Universidade de São Paulo, Av. dos Bandeirantes 3900, 14040–901 Ribeirão Preto, SP, Brazil. [email protected] (RS), [email protected] (FAB) 4 Institut de Systématique, Évolution, Biodiversité, ISYEB – UMR 7205 – CNRS, MNHN, UPMC, EPHE, Muséum national d’Histoire naturelle, Sorbonne Universités, 25 rue Cuvier, CP 30, 75005, Paris, France. [email protected] (AD). 5 Departamento de Zoologia, Instituto de Biologia, Universidade Federal do Rio de Janeiro, Av. Carlos Chagas Filho, 373, 21941- 902, Rio de Janeiro, RJ, Brazil. 6 Programa de Pós-Graduação em Biologia Comparada, FFCLRP, Universidade de São Paulo, Av. -

Cop18 Prop. 39
Original language: English CoP18 Prop. 39 CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________________ Eighteenth meeting of the Conference of the Parties Colombo (Sri Lanka), 23 May – 3 June 2019 CONSIDERATION OF PROPOSALS FOR AMENDMENT OF APPENDICES I AND II A. Proposal Inclusion of Echinotriton chinhaiensis (Chang, 1932) and Echinotriton maxiquadratus Hou, Wu, Yang, Zheng, Yuan, and Li, 2014, both of which are endemic to China in Appendix Ⅱ, in accordance with Article Ⅱ, paragraph 2 (a) of the Convention and satisfying Criterion B in Annex 2a of Resolution Conf. 9.24 (Rev. CoP17). The international trade of these two newts should be monitored to minimise the impact of illegal hunting driven by international pet trade or collection on the survival of these two critically endangered species B. Proponent China*: C. Supporting statement 1. Taxonomy 1.1 Class: Amphibia 1.2 Order: Caudata 1.3 Family: Salamandridae 1.4 Genus, species or subspecies, including author and year: 1.5 Scientific synonyms: Echinotriton chinhaiensis: Tylototriton chinhaiensis Chang, 1932; Tylototriton (Echinotriton) chinhaiensis; Pleurodeles chinhaiensis (Chang, 1932); Pleurodeles (Tylototrion) chinhaiensis 1.6 Common names: English: E. chinhaiensis: Chinhai Spiny Newt, Chinhai Spiny Crocodile Newt E. maxiquadratus: Mountain Spiny Newt, Mountain Spiny Crocodile Newt French: Spanish: 1.7 Code numbers: N/A * The geographical designations employed in this document do not imply the expression of any opinion whatsoever on the part of the CITES Secretariat (or the United Nations Environment Programme) concerning the legal status of any country, territory, or area, or concerning the delimitation of its frontiers or boundaries. The responsibility for the contents of the document rests exclusively with its author. -

Western Tiger Salamander,Ambystoma Mavortium
COSEWIC Assessment and Status Report on the Western Tiger Salamander Ambystoma mavortium Southern Mountain population Prairie / Boreal population in Canada Southern Mountain population – ENDANGERED Prairie / Boreal population – SPECIAL CONCERN 2012 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2012. COSEWIC assessment and status report on the Western Tiger Salamander Ambystoma mavortium in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xv + 63 pp. (www.registrelep-sararegistry.gc.ca/default_e.cfm). Previous report(s): COSEWIC. 2001. COSEWIC assessment and status report on the tiger salamander Ambystoma tigrinum in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 33 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Schock, D.M. 2001. COSEWIC assessment and status report on the tiger salamander Ambystoma tigrinum in Canada, in COSEWIC assessment and status report on the tiger salamander Ambystoma tigrinum in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-33 pp. Production note: COSEWIC would like to acknowledge Arthur Whiting for writing the status report on the Western Tiger Salamander, Ambystoma mavortium, in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Kristiina Ovaska, Co-chair of the COSEWIC Amphibians and Reptiles Specialist Subcommittee. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur la Salamandre tigrée de l’Ouest (Ambystoma mavortium) au Canada. -

Notophthalmus Perstriatus) Version 1.0
Species Status Assessment for the Striped Newt (Notophthalmus perstriatus) Version 1.0 Striped newt eft. Photo credit Ryan Means (used with permission). May 2018 U.S. Fish and Wildlife Service Region 4 Jacksonville, Florida 1 Acknowledgements This document was prepared by the U.S. Fish and Wildlife Service’s North Florida Field Office with assistance from the Georgia Field Office, and the striped newt Species Status Assessment Team (Sabrina West (USFWS-Region 8), Kaye London (USFWS-Region 4) Christopher Coppola (USFWS-Region 4), and Lourdes Mena (USFWS-Region 4)). Additionally, valuable peer reviews of a draft of this document were provided by Lora Smith (Jones Ecological Research Center) , Dirk Stevenson (Altamaha Consulting), Dr. Eric Hoffman (University of Central Florida), Dr. Susan Walls (USGS), and other partners, including members of the Striped Newt Working Group. We appreciate their comments, which resulted in a more robust status assessment and final report. EXECUTIVE SUMMARY This Species Status Assessment (SSA) is an in-depth review of the striped newt's (Notophthalmus perstriatus) biology and threats, an evaluation of its biological status, and an assessment of the resources and conditions needed to maintain species viability. We begin the SSA with an understanding of the species’ unique life history, and from that we evaluate the biological requirements of individuals, populations, and species using the principles of population resiliency, species redundancy, and species representation. All three concepts (or analogous ones) apply at both the population and species levels, and are explained that way below for simplicity and clarity as we introduce them. The striped newt is a small salamander that uses ephemeral wetlands and the upland habitat (scrub, mesic flatwoods, and sandhills) that surrounds those wetlands. -

The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom
lopmen ve ta e l B Williamson, Cell Dev Biol 2012, 1:1 D io & l l o l g DOI: 10.4172/2168-9296.1000101 e y C Cell & Developmental Biology ISSN: 2168-9296 Research Article Open Access The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom Abstract The larval transfer hypothesis states that larvae originated as adults in other taxa and their genomes were transferred by hybridization. It contests the view that larvae and corresponding adults evolved from common ancestors. The present paper reviews the life histories of chordates, and it interprets them in terms of the larval transfer hypothesis. It is the first paper to apply the hypothesis to craniates. I claim that the larvae of tunicates were acquired from adult larvaceans, the larvae of lampreys from adult cephalochordates, the larvae of lungfishes from adult craniate tadpoles, and the larvae of ray-finned fishes from other ray-finned fishes in different families. The occurrence of larvae in some fishes and their absence in others is correlated with reproductive behavior. Adult amphibians evolved from adult fishes, but larval amphibians did not evolve from either adult or larval fishes. I submit that [1] early amphibians had no larvae and that several families of urodeles and one subfamily of anurans have retained direct development, [2] the tadpole larvae of anurans and urodeles were acquired separately from different Mesozoic adult tadpoles, and [3] the post-tadpole larvae of salamanders were acquired from adults of other urodeles. Reptiles, birds and mammals probably evolved from amphibians that never acquired larvae. -

Caudata: Hynobiidae): Heterochronies and Reductions
65 (1): 117 – 130 © Senckenberg Gesellschaft für Naturforschung, 2015. 4.5.2015 Development of the bony skeleton in the Taiwan salamander, Hynobius formosanus Maki, 1922 (Caudata: Hynobiidae): Heterochronies and reductions Anna B. Vassilieva 1 *, June-Shiang Lai 2, Shang-Fang Yang 2, Yu-Hao Chang 1 & Nikolay A. Poyarkov, Jr. 1 1 Department of Vertebrate Zoology, Biological Faculty, Lomonosov Moscow State University, Leninskiye Gory, GSP-1, Moscow 119991, Russia — 2 Department of Life Science, National Taiwan Normal University, 88, Sec. 4 Tingchou Rd., Taipei 11677, Taiwan, R.O.C. — *Cor- responding author; vassil.anna(at)gmail.com Accepted 19.ii.2015. Published online at www.senckenberg.de / vertebrate-zoology on 4.v.2015. Abstract The development of the bony skeleton in a partially embryonized lotic-breeding salamander Hynobius formosanus is studied using the ontogenetic series from late embryos to postmetamorphic juveniles and adult specimen. Early stages of skull development in this spe- cies are compared with the early cranial ontogeny in two non-embryonized lentic-breeding species H. lichenatus and H. nigrescens. The obtained results show that skeletal development distinguishes H. formosanus from other hynobiids by a set of important features: 1) the reduction of provisory ossifications (complete absence of palatine and reduced state of coronoid), 2) alteration of a typical sequence of ossification appearance, namely, the delayed formation of vomer and coronoid, and 3) the absence of a separate ossification center of a lacrimal and formation of a single prefrontolacrimal. These unique osteological characters in H. formosanus are admittedly connected with specific traits of its life history, including partial embryonization, endogenous feeding until the end of metamorphosis and relatively short larval period. -

Amphibiaweb's Illustrated Amphibians of the Earth
AmphibiaWeb's Illustrated Amphibians of the Earth Created and Illustrated by the 2020-2021 AmphibiaWeb URAP Team: Alice Drozd, Arjun Mehta, Ash Reining, Kira Wiesinger, and Ann T. Chang This introduction to amphibians was written by University of California, Berkeley AmphibiaWeb Undergraduate Research Apprentices for people who love amphibians. Thank you to the many AmphibiaWeb apprentices over the last 21 years for their efforts. Edited by members of the AmphibiaWeb Steering Committee CC BY-NC-SA 2 Dedicated in loving memory of David B. Wake Founding Director of AmphibiaWeb (8 June 1936 - 29 April 2021) Dave Wake was a dedicated amphibian biologist who mentored and educated countless people. With the launch of AmphibiaWeb in 2000, Dave sought to bring the conservation science and basic fact-based biology of all amphibians to a single place where everyone could access the information freely. Until his last day, David remained a tirelessly dedicated scientist and ally of the amphibians of the world. 3 Table of Contents What are Amphibians? Their Characteristics ...................................................................................... 7 Orders of Amphibians.................................................................................... 7 Where are Amphibians? Where are Amphibians? ............................................................................... 9 What are Bioregions? ..................................................................................10 Conservation of Amphibians Why Save Amphibians? ............................................................................. -
Comparative Osteology and Evolution of the Lungless Salamanders, Family Plethodontidae David B
COMPARATIVE OSTEOLOGY AND EVOLUTION OF THE LUNGLESS SALAMANDERS, FAMILY PLETHODONTIDAE DAVID B. WAKE1 ABSTRACT: Lungless salamanders of the family Plethodontidae comprise the largest and most diverse group of tailed amphibians. An evolutionary morphological approach has been employed to elucidate evolutionary rela tionships, patterns and trends within the family. Comparative osteology has been emphasized and skeletons of all twenty-three genera and three-fourths of the one hundred eighty-three species have been studied. A detailed osteological analysis includes consideration of the evolution of each element as well as the functional unit of which it is a part. Functional and developmental aspects are stressed. A new classification is suggested, based on osteological and other char acters. The subfamily Desmognathinae includes the genera Desmognathus, Leurognathus, and Phaeognathus. Members of the subfamily Plethodontinae are placed in three tribes. The tribe Hemidactyliini includes the genera Gyri nophilus, Pseudotriton, Stereochilus, Eurycea, Typhlomolge, and Hemidac tylium. The genera Plethodon, Aneides, and Ensatina comprise the tribe Pleth odontini. The highly diversified tribe Bolitoglossini includes three super genera. The supergenera Hydromantes and Batrachoseps include the nominal genera only. The supergenus Bolitoglossa includes Bolitoglossa, Oedipina, Pseudoeurycea, Chiropterotriton, Parvimolge, Lineatriton, and Thorius. Manculus is considered to be congeneric with Eurycea, and Magnadig ita is congeneric with Bolitoglossa. Two species are assigned to Typhlomolge, which is recognized as a genus distinct from Eurycea. No. new information is available concerning Haptoglossa. Recognition of a family Desmognathidae is rejected. All genera are defined and suprageneric groupings are defined and char acterized. Range maps are presented for all genera. Relationships of all genera are discussed. -

Zootaxa, a New Species of Paramesotriton (Caudata
Zootaxa 1775: 51–60 (2008) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2008 · Magnolia Press ISSN 1175-5334 (online edition) A new species of Paramesotriton (Caudata: Salamandridae) from Guizhou Province, China HAITAO ZHAO1, 2, 5, JING CHE2,5, WEIWEI ZHOU2, YONGXIANG CHEN1, HAIPENG ZHAO3 & YA-PING ZHANG2 ,4 1Department of Environment and Life Science, Bijie College, Guizhou 551700, China 2State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, the Chinese Academy of Sciences, Kunming 650223, China 3School of Life Science, Southwest University, Chongqing 400715, China 4Corresponding authors. E-mail: [email protected] 5 These authors contributed equally to this work. Abstract We describe a new species of salamander, Paramesotriton zhijinensis, from Guizhou Province, China. The generic allo- cation of the new species is based on morphological and molecular characters. In morphology, it is most similar to Paramesotriton chinensis but differs in having distinct gland emitting a malodorous secretion (here named scent gland), a postocular stripe, and two non-continuous, dorsolateral stripes on the dorsolateral ridges. Furthermore, neoteny was observed in most individuals of the new species. This has not been previously reported to occur in any other species of Paramesotriton. Analysis of our molecular data suggests that this species a third major evolutionary lineage in the genus Paramesotriton. Key words: Caudata; Salamandridae; Paramesotriton zhijinensis; new species; scent gland; Guizhou; China Introduction Guizhou Province, located in the southwestern mountainous region of China, is known for its rich amphibian faunal diversity (Liu and Hu 1961). During recent surveys of the Guizhou herpetofauna (July, September, and November, 2006; January and September, 2007), we collected salamanders superficially resembling Parame- sotriton chinensis (Gray). -

Successful Reproduction of the Mole Salamander Ambystoma Talpoideum in Captivity, with an Emphasis on Stimuli Environmental Determinants
SHORT NOTE The Herpetological Bulletin 141, 2017: 28-31 Successful reproduction of the mole salamander Ambystoma talpoideum in captivity, with an emphasis on stimuli environmental determinants AXEL HERNANDEZ Department of Environmental Sciences, Faculty of Sciences and Technics, University Pasquale Paoli of Corsica, Corte, 20250, France Author Email: [email protected] ABSTRACT - Generating and promoting evidence-based husbandry protocols for urodeles, commonly known as newts and salamanders, is urgently needed because most of the up-to-date ex situ programs are focused on frogs and toads than Urodela. Data on biology, life history, ecology and environmental parameters are lacking for many species and are needed to establish suitable husbandry and breeding conditions in captive environments. Two adult females and two adult males, of the mole salamander Ambystoma talpoideum successfully reproduced in captivity. It was found that reproduction of this species depends on various complex stimuli: including natural photoperiod 12:12, rainwater (acidic to neutral pH) and an aquarium full of various debris. Additionally high temperature variations ranging from 2 °C to 17 °C (a decrease followed by an increase) between November and February showed that it is possible to breed adults in aquariums provided the right stimuli are applied at the right moment of time in winter. A. talpoideum shows an explosive breeding mode as previously reported for the whole genus Ambystoma. INTRODUCTION with an emphasis on the environmental determinant stimuli involved. These data may assist in improving breeding these ince the 1980s, the current global amphibian extinction salamanders under artificial conditions. crisis has been discussed and acknowledged (Wake, A. -

35 Finding of the Alpine Salamander (Salamandra Atra Laurenti, 1768; Salamandridae, Caudata) in the Nature Park Žumberak
HYLA VOL. 2011. No. 1 Str. 35-46 SHORT NOTE Finding of the Alpine salamander (Salamandra atra Laurenti, 1768; Salamandridae, Caudata) in the Nature Park Žumberak - Samoborsko gorje (NW Croatia) 1 2 3 NINA JERAN* , PETRA ĐURIĆ , KREŠIMIR ŽGANEC 1 Požarinje 69, 10000 Zagreb, Croatia, [email protected] 2 State Institute for Nature Protection, Trg Mažuranića 5, 10000 Zagreb, Croatia 3 University of Zagreb, Faculty of Science, Rooseveltov trg 6, 10000 Zagreb, Croatia ABSTRACT This study confirms the presence of Alpine salamander (Salamandra atra) in the Nature Park Žumberak - Samoborsko gorje, where previously only one specimen was recorded in 1989. Species presence and distribution were investigated at ten different localities in stands of montane beech forest, during the vegetation season 2004. In July 2004 five individuals (four males and one female) of Alpine salamander were found in the virgin beech forest at site Kuta (about 900 m a.s.l.), during weather conditions characterized by heavy rain. This is the northernmost finding of the species in Croatia, as well as a confirmed disjunctive part of its areal in the Dinarids. Conservation measures for the species are proposed but for more precise conservation plan further research of species distribution and ecology is needed. Keywords: Alpine salamander, Salamandra atra, Nature Park Žumberak - Samoborsko gorje, distribution, amphibians 35 Alpine salamander (Salamandra atra) is a montane species occurring between 400 and 2,800 m a.s.l., but is usually found from 800 to 2,000 m a.s.l. (ARNOLD & BURTON, 1978). It inhabits mainly forests (beech, mixed deciduous and mixed deciduous-coniferous), but may also be found above the tree-line, in cool, damp alpine meadows, pastureland and other, slightly modified habitats.