Cercopithecus Diana) in Côte D’Ivoire’S Taï National Park
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
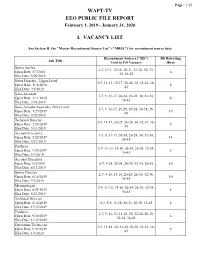
Wapt-Tv Eeo Public File Report I. Vacancy List
Page: 1/15 WAPT-TV EEO PUBLIC FILE REPORT February 1, 2019 - January 31, 2020 I. VACANCY LIST See Section II, the "Master Recruitment Source List" ("MRSL") for recruitment source data Recruitment Sources ("RS") RS Referring Job Title Used to Fill Vacancy Hiree Sports Anchor 2-3, 5-13, 15-16, 20-21, 23-28, 30, 32- Open Date: 5/7/2018 6 34, 36-45 Hire Date: 5/29/2019 News Director - Upper Level 2-9, 11-13, 15-17, 20-28, 30, 32-34, 36- Open Date: 11/6/2018 8 45 Hire Date: 3/3/2019 Sales Assistant 1-7, 9, 11-17, 20-24, 26-28, 30, 32-34, Open Date: 1/11/2019 6 36-45 Hire Date: 3/18/2019 Sales Account Associate- Entry Level 1-7, 9, 11-17, 19-24, 26-28, 30-34, 36- Open Date: 1/29/2019 19 45 Hire Date: 5/20/2019 Technical Director 2-9, 11-17, 20-24, 26-28, 30, 32-34, 36- Open Date: 2/25/2019 8 45 Hire Date: 5/13/2019 Account Executive 1-7, 9, 11-17, 20-24, 26-28, 30, 32-34, Open Date: 2/28/2019 14 36-45 Hire Date: 5/27/2019 Producer 2-9, 11-13, 15-18, 20-24, 26-30, 32-34, Open Date: 4/26/2019 8 36-45 Hire Date: 9/1/2019 Account Executive Open Date: 6/5/2019 1-7, 9-18, 20-24, 26-30, 32-34, 36-45 10 Hire Date: 8/12/2019 Sports Director 2-7, 9-13, 15-18, 20-24, 26-30, 32-34, Open Date: 6/18/2019 10 36-45 Hire Date: 9/3/2019 Meteorologist 2-9, 11-13, 15-18, 20-24, 26-30, 32-34, Open Date: 6/21/2019 8 36-45 Hire Date: 8/23/2019 Technical Director Open Date: 8/12/2019 2-3, 5-9, 11-18, 20-23, 26-30, 32-45 8 Hire Date: 11/11/2019 Producer 1-7, 9, 11-13, 15-18, 20, 22-24, 26-30, Open Date: 9/30/2019 6 32-34, 36-45 Hire Date: 11/11/2019 Operations Technician 2-9, 11-18, 20, 22-24, 26-30, 32-34, 36- Open Date: 9/30/2019 8 45 Hire Date: 1/3/2020 Page: 2/15 WAPT-TV EEO PUBLIC FILE REPORT February 1, 2019 - January 31, 2020 II. -

University of Ghana College of Basic and Applied
UNIVERSITY OF GHANA COLLEGE OF BASIC AND APPLIED SCIENCES DEPARTMENT OF NUTRITION AND FOOD SCIENCE CHEMICAL COMPOSITION AND ANTIMICROBIAL EFFICACY OF ESSENTIAL OIL FROM XYLOPIA AETHIOPICA FRUIT PODS BY DZESI KWAME TORKU (10551784) THIS THESIS IS SUBMITTED TO THE UNIVERSITY OF GHANA, LEGON IN PARTIAL FULFILMENT OF THE REQUIREMENT FOR THE AWARD OF MPHIL FOOD SCIENCE DEGREE JULY 2017 DECLARATION I declare that this thesis was carried out by me under supervision in the Department of Nutrition and Food Science, University of Ghana, Legon. I take full responsibility for whatever has been reported here. Related work by others, which served as a source of information has been duly acknowledged by reference to the authors. -------------------------------------------- ------------------------------ TORKU DZESI KWAME DATE (STUDENT) ---------------------------------------------- ----------------------------- DR ANGELA PARRY-HANSON KUNADU DATE (PRINCIPAL SUPERVISOR) -------------------------------------------- ------------------------------ DR MAAME YAAKWAAH BLAY ADJEI DATE (CO - SUPERVISOR) -------------------------------------------- ------------------------------ PROF. FIRIBU KWESI SAALIA DATE (CO - SUPERVISOR) i DEDICATION This research work is dedicated to God Almighty, my dad Mr Benjamin Sefakor Cudjo Torku (of blessed memory), all my siblings, my mother Comfort Yevuyibor Stella Afua (Mrs. Torku), my bosom friend Mr Alexander Kwame Gavu and all who supported me financially during my study. ii ACKNOWLEDGEMENT I am grateful to God Almighty for his favour granted me throughout my research work. I earnestly appreciate my supervisors, Dr Angela Parry-Hanson Kunadu, Dr Maame Yaakwaah Blay Adjei and Prof. Firibu Kwesi Saalia for their intellectual support and motivation without which I could not have completed my research work. I am happy to have benefited from the Essential Oils Project at the Department of Nutrition and Food Science, University of Ghana, a project funded by the Office of Research, Innovation and Development, University of Ghana. -

World's Most Endangered Primates
Primates in Peril The World’s 25 Most Endangered Primates 2016–2018 Edited by Christoph Schwitzer, Russell A. Mittermeier, Anthony B. Rylands, Federica Chiozza, Elizabeth A. Williamson, Elizabeth J. Macfie, Janette Wallis and Alison Cotton Illustrations by Stephen D. Nash IUCN SSC Primate Specialist Group (PSG) International Primatological Society (IPS) Conservation International (CI) Bristol Zoological Society (BZS) Published by: IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), Bristol Zoological Society (BZS) Copyright: ©2017 Conservation International All rights reserved. No part of this report may be reproduced in any form or by any means without permission in writing from the publisher. Inquiries to the publisher should be directed to the following address: Russell A. Mittermeier, Chair, IUCN SSC Primate Specialist Group, Conservation International, 2011 Crystal Drive, Suite 500, Arlington, VA 22202, USA. Citation (report): Schwitzer, C., Mittermeier, R.A., Rylands, A.B., Chiozza, F., Williamson, E.A., Macfie, E.J., Wallis, J. and Cotton, A. (eds.). 2017. Primates in Peril: The World’s 25 Most Endangered Primates 2016–2018. IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society, Arlington, VA. 99 pp. Citation (species): Salmona, J., Patel, E.R., Chikhi, L. and Banks, M.A. 2017. Propithecus perrieri (Lavauden, 1931). In: C. Schwitzer, R.A. Mittermeier, A.B. Rylands, F. Chiozza, E.A. Williamson, E.J. Macfie, J. Wallis and A. Cotton (eds.), Primates in Peril: The World’s 25 Most Endangered Primates 2016–2018, pp. 40-43. IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society, Arlington, VA. -

Download Download
Medicinal uses of plants by traditional birth attendants to facilitate childbirth among Djimini women in Dabakala (Center-North of Côte d'Ivoire) Silvère Romuald Koman, Wokapeu Blaise Kpan, Konan Yao and Djakalia Ouattara Research Blighia sapida are the plants most used for their oxytocic effect in the department of Dabakala. Abstract Conclusion: These results constitute a database for Background: Today, due to the still high rates of subsequent studies aimed at assessing the maternal death and incessant caesareans, the high biological and chemical composition of these plants. cost of pharmaceutical drugs, parturients are turning to traditional midwives to facilitate childbirth. The Keywords: medicinal plants, traditional midwives, purpose of this study is to identify and document the childbirth, Côte d’Ivoire medicinal plants traditionally used for their oxytocic effect by the traditional midwives in the department Correspondence of Dabakala. 1 Methods: an ethnobotanical survey was conducted Silvère Romuald Koman , Wokapeu Blaise 1,2,3 2,3,4 1,2 with 71 traditional midwives from the department of Kpan , Konan Yao and Djakalia Ouattara DabaKala. The data were analyzed with Epidata and 1 SPSS 20.0 software. The Chi-square test was used Laboratory of Natural Environments and to compare the percentages of the parts used, the Biodiversity Conservation, Botanical Research and method of preparation and the method of Training Unit, Félix Houphouët-Boigny University, administration. To better interpret the cultural 22 BP 582 Abidjan 22 2 medicinal -

Therapeutic and Ameliorative Effects of Active Compounds of Combretum Molle in the Treatment and Relief from Wounds in a Diabetes Mellitus Experimental Model
coatings Article Therapeutic and Ameliorative Effects of Active Compounds of Combretum molle in the Treatment and Relief from Wounds in a Diabetes Mellitus Experimental Model Reham Z. Hamza 1,*, Shaden E. Al-Motaani 2 and Tarek Al-Talhi 3 1 Biology Department, College of Sciences, Taif University, Taif—P.O. Box 11099, Taif 21944, Saudi Arabia 2 General Department of Education, Taif 21944, Saudi Arabia; [email protected] 3 Chemistry Department, College of Sciences, Taif University, Taif—P.O. Box 11099, Taif 21944, Saudi Arabia; [email protected] * Correspondence: [email protected] or [email protected] Abstract: Foot ulcers are one of the leading causes of severe and high mortality in diabetics. It is known that wound healing in diabetics is a very complicated process due to the direct severe effect of diabetes mellitus on blood vessels, causing difficulty in wound healing. Many methods of treatment have recently been employed for novel dressings for the promotion of tissue regeneration and rapid wound closure. Combretum molle is composed of chemical compounds, such as lignin, gallic acid, and ellagic acid. Twenty male rats that were 4 months of age were divided into a I-a diabetic foot ulcer group as the control group and a II-a diabetic group (wound + Combretum molle). This study investigated the antioxidant and excellent healing effects of the extract of Combretum molle in repairing Citation: Hamza, R.Z.; Al-Motaani, skin damaged by diabetes. This was confirmed by elevated antioxidant enzymes in the animals’ S.E.; Al-Talhi, T. Therapeutic and tissues in diabetic rats treated with this extract. -
Northsidesun
the weekly $20 For A Year. Call 957-1122 www.northsidesun.com 10,120 Paid Circulation; 25.300 Readership For 41 Years, Covering Northeastnorthside Jackson, Madison and Ridgeland Two Sections, 20 Pages,sun 75 Cents, Thursday, July 9, 2009 MILLIONS NEEDED FOR UPGRADE Age causing havoc with infrastructure By ANTHONY WARREN repairs. sive sinkhole under Ridgewood Road and storm drain are usually last on the list, while Sun Staff Writer “Normally, during a dry period, we average blocked two lanes of traffic for several months. breaks like the ones that occurred on YEARS OF EXPOSURE to the elements about five breaks a day,” said Public Works Fortification and Old Canton are top priority. took its toll on more Jackson infrastructure Deputy Director David Willis. “Most of the MUCH OF the 42-inch main crumbled, forc- Once a rupture is identified, the city contacts recently when two water mains ruptured under time, we can put a repair clamp on the break, ing the city to spend $1.3 million on a special Mississippi 811, formerly Mississippi One Call, roadways on the Northside. but when the pipe completely breaks, we have “sock” to reinforce what was left of the line. a service that helps crews locate underground Last week, water lines burst under to cut out the damaged portion and install a new City engineers say the sock, once it hardened, utilities and gas lines before they dig. That Fortification Street and Old Canton Road, send- section.” will last for 40 years. After repairs were made to process, Willis said, usually takes about four ing mounds of asphalt flying through the air and New sections of pipe had to be installed at that project, another sinkhole formed about 150 hours. -

Liza Cirlot Looser Chairman and Chief Executive Officer
LIZA CIRLOT LOOSER CHAIRMAN AND CHIEF EXECUTIVE OFFICER Liza is the founder and CEO of The Cirlot Agency, a global brand strategy, integrated communications and business development firm, with offices in Jackson, MS, and Washington, DC. Over the past 35 years, the firm has grown to represent publicly traded companies and privately held corporations on a national and international basis. It has also become known as one of the leading aerospace defense communications agencies in the nation, having worked in the industry for 35 years. A graduate of Mississippi University for Women and Harvard Business School, Liza has expanded her studies to include several international business fellowships and special assignments. In 2011, Liza was named chairman of the board of the Mississippi World Trade Center and was awarded the organization’s highest honor, the Tozzoli International Business Leadership Award. As a testament to her international business acumen, Liza’s experience includes representation of clients in Europe, South America and the Middle East. A career highlight in 1998, Liza was asked by NATO to help devise a communications plan regarding the Bosnian land mine crisis to give the citizens of Bosnia a voice, and worked with Her Majesty’s Engineer in England to facilitate the plan. Liza began her career as an advertising account executive at The Clarion-Ledger, the largest daily newspaper in the state of Mississippi. From there, Liza worked for what was then the state’s largest advertising agency and spent time in the political arena during the Reagan/Bush era working as Communications Director of the state’s Republican Party. -

Antifungal Activity of Xylopia Aethiopica on Some Clinical Organisms in Nigeria
Hamdard Medicus Vol. 55, No. 1, 2012 Antifungal Activity of Xylopia aethiopica on Some Clinical Organisms in Nigeria U.C. Kanife*, F. Doherty, N.M.C. Nwakanma and G.O.L. Adamu Department of Biological Science, School of Science, Yaba College of Technology, Yaba, Lagos, Nigeria. The antifungal properties of water, ethanol and chloroform extracts of the fruits of Xylopia aethiopica were investigated using agar well diffusion method. Aqueous extract was not potent against all the organisms tested. Chloroform and ethanol extracts were active against Candida albicans and Aspergillus niger at different concentrations (50 mg/ml, 75 mg/ml and 100 mg/ml) with varying zones of inhibition. The chloroform extracts of Xylopia aethiopica had the highest zone of inhibition of 5.4 mm at 75 mg/ml which is comparable to that of Nystatin. Ethanol extract also had strongest inhibition at 75 mg/ml with 5.0 mm mean zone of inhibition against Aspergillus niger. The crude extracts of Xylopia aethiopica contained Saponins, Flavonoids, Phenol, Alkaloids and Terpenoids. Keywords: Antifungal activity, Crude extract, Xylopia aethiopica. Introduction Xylopia aethiopica is an angiosperm belonging to the family Annonaceae used mainly as spices and in traditional medicine. A tree of 20 m height or more with a clear straight bole in 75 cm girth, often with short prop roots, smooth grey bark and scented when fresh (Boakye-Yiadom et al., 1971). The fruit is the most important part of the tree, it is narrow, dark brown or black about 2 inches long with separate carpel on a stout peduncle. The plant is distributed in all forest regions of Senegal to Zaire and has been located in Gambia, Nigeria, Gabon, Ivory Coast, Togo and Tanzania (Day et al., 1990). -

Mt Mabu, Mozambique: Biodiversity and Conservation
Darwin Initiative Award 15/036: Monitoring and Managing Biodiversity Loss in South-East Africa's Montane Ecosystems MT MABU, MOZAMBIQUE: BIODIVERSITY AND CONSERVATION November 2012 Jonathan Timberlake, Julian Bayliss, Françoise Dowsett-Lemaire, Colin Congdon, Bill Branch, Steve Collins, Michael Curran, Robert J. Dowsett, Lincoln Fishpool, Jorge Francisco, Tim Harris, Mirjam Kopp & Camila de Sousa ABRI african butterfly research in Forestry Research Institute of Malawi Biodiversity of Mt Mabu, Mozambique, page 2 Front cover: Main camp in lower forest area on Mt Mabu (JB). Frontispiece: View over Mabu forest to north (TT, top); Hermenegildo Matimele plant collecting (TT, middle L); view of Mt Mabu from abandoned tea estate (JT, middle R); butterflies (Lachnoptera ayresii) mating (JB, bottom L); Atheris mabuensis (JB, bottom R). Photo credits: JB – Julian Bayliss CS ‒ Camila de Sousa JT – Jonathan Timberlake TT – Tom Timberlake TH – Tim Harris Suggested citation: Timberlake, J.R., Bayliss, J., Dowsett-Lemaire, F., Congdon, C., Branch, W.R., Collins, S., Curran, M., Dowsett, R.J., Fishpool, L., Francisco, J., Harris, T., Kopp, M. & de Sousa, C. (2012). Mt Mabu, Mozambique: Biodiversity and Conservation. Report produced under the Darwin Initiative Award 15/036. Royal Botanic Gardens, Kew, London. 94 pp. Biodiversity of Mt Mabu, Mozambique, page 3 LIST OF CONTENTS List of Contents .......................................................................................................................... 3 List of Tables ............................................................................................................................. -

Grains of Selim) As Additive
DOI: 10.2478/ats-2014-0017 AGRICULTURA TROPICA ET SUBTROPICA, 47/4, 124-130, 2014 Original Research Article Performance, Antimicrobial Effect and Carcass Parameters of Finisher Broilers Given Xylopia aethiopica Dried Fruits (Grains of Selim) as Additive Jonathan Ogagaoghene Isikwenu, Ifeanyi Udeh, Bernard Izuchukwu Oshai, Theresa Ogheneremu Kekeke Department of Animal Science, Delta State University, Asaba Campus, Nigeria Abstract The effect of graded levels of grains of selim on the performance, gut microbial population and carcass characteristics of finisher broilers was investigated. Two hundred and four (204) 28 days old broiler chicks (Marshal breed) were randomly allotted to four treatments with each treatment having three replicates of 17 chicks each in a completely randomized design. Finely blended grains of selim was administered through drinking water on treatments 2, 3 and 4 at concentrations of 0.5, 0.7 and 0.9 g per litre while chicks on treatment 1 (control) received 1.0 g per 1.5 litre of antibiotics (Gendox). Chicks were fed ad libitum with isonitrogenous and isocaloric diets containing 20% crude protein and 3000 Kcal/kg metabolisable energy for four weeks. Results showed no significant (P > 0.05) differences among treatments in the final body weight, total weight gain, daily weight gain, total feed intake, daily feed intake and feed:gain ratio. There were differences in the microbial population of the gastro-intestinal tract with colony count decreasing as the concentration of grains of selim increases. Carcass characteristics and organ weights were similar (P > 0.05) except for thigh and spleen weights, and large intestine lengths where differences (P < 0.05) exist. -

A PERFECT WAY to OBTAIN RECOGNITION for YOUR 1) Fill out the Order Form – 2) Fill out the Inscription Form and Return To: DONATION to THE
2918 West Capitol Street 2918 West Jackson, MS 39209 ORDER NOW! Don’t miss out on having your name 601-352-2580 inscribed at the Jackson Zoo! A PERFECT WAY TO OBTAIN RECOGNITION FOR YOUR 1) Fill out the Order Form – 2) Fill out the Inscription form and return to: DONATION TO THE Jackson Zoo JACKSON ZOO! Donor Tile Program 2918 West Capitol Street Jackson, MS 39209 MY ORDER Check Payable to: The Jackson Zoo Visa MasterCard Card Number Exp. Date NAME: ADDRESS: CITY: STATE: ZIP: PHONE: EMAIL: Indicate Selection: 12” x 12” Animal Tile at $1,000 = Tiger Orangutan 8” x 8” Inscription Tile at $750 = 6” x 6” Animal Tile at $500 = Leopard Gibbon 3” x 6” Inscription Tile at $200 = Org Non-Prot U.S. Postage Postage U.S. Permit #178 Permit Jackson, MS PAID Total Amount INSCRIPTION FORM – PRINT CAREFULLY! hese tiles will be installed on a beautiful wall structure which will be part of the work being completed in the Asia section of the Jackson Zoo, along with a new 12” x 12” Animal Tile T (up to 4-line inscription) exhibit for tigers, renovated exhibits for Amur leopards, 33 CHARACTERS PER LINE and for gibbons, and the already completed orangutan (INCLUDING SPACES & exhibit. PUNCTUATION) The first tiles will start going up at the end of 2008, and will serve as a reminder to the next generations of visitors of your support. 8” x 8” Inscription Tile (up to 4-line inscription) 30 CHARACTERS PER LINE (INCLUDING SPACES & PUNCTUATION) Examples Only By donating and investing in a tile at the Jackson Zoo, you will be contributing to ongoing development of our zoo. -

Rare Amur Leopard Birth at Jackson Zoo
NEWS 2918 West Capitol Street Jackson, MS 39209 Date: August 30, 2007 Contact: Christopher Mims, Director of Marketing and Public Relations 601-352-2599 Rare Amur Leopard Birth at Jackson Zoo The Jackson Zoo is pleased to announce the birth of a female Amur Leopard, Judda, who turned six weeks old Wednesday. The Jackson Zoo has been home to two Amur Leopards, Nikolai (male) and Katya (female), since 2004. This was Katya’s first pregnancy, and of the two offspring born, only one survived. The surviving cub has had to be hand-fed by Zoo staffers since birth and is not currently on display. “Sometimes new mommies can be a little rough with newborns, and we felt that Judda needed to be observed very closely,” said Vet. Tech Donna Todd. Amur leopards are very distinct from other leopards. Their long legs and long hair allow them to survive in cold, snowy climates. Their spots are widely spaced rosettes with thick borders. The color of their fur changes from lighter in the winter to a more reddish-yellow in the summer. The average adult male weighs between 82-198 pounds and the average female weighs between 62-132 pounds. Photo: Judda practices her ferocious snarl. Amur Leopards are critically endangered with field survey data estimates indicating that there are fewer than 50 leopards left in the wild, making the Amur leopard one of the world’s most endangered cats. The latest results estimated there were 25-40 Amur leopards in the Russian Far East and 4-7 in northeast China (Jilin Province).