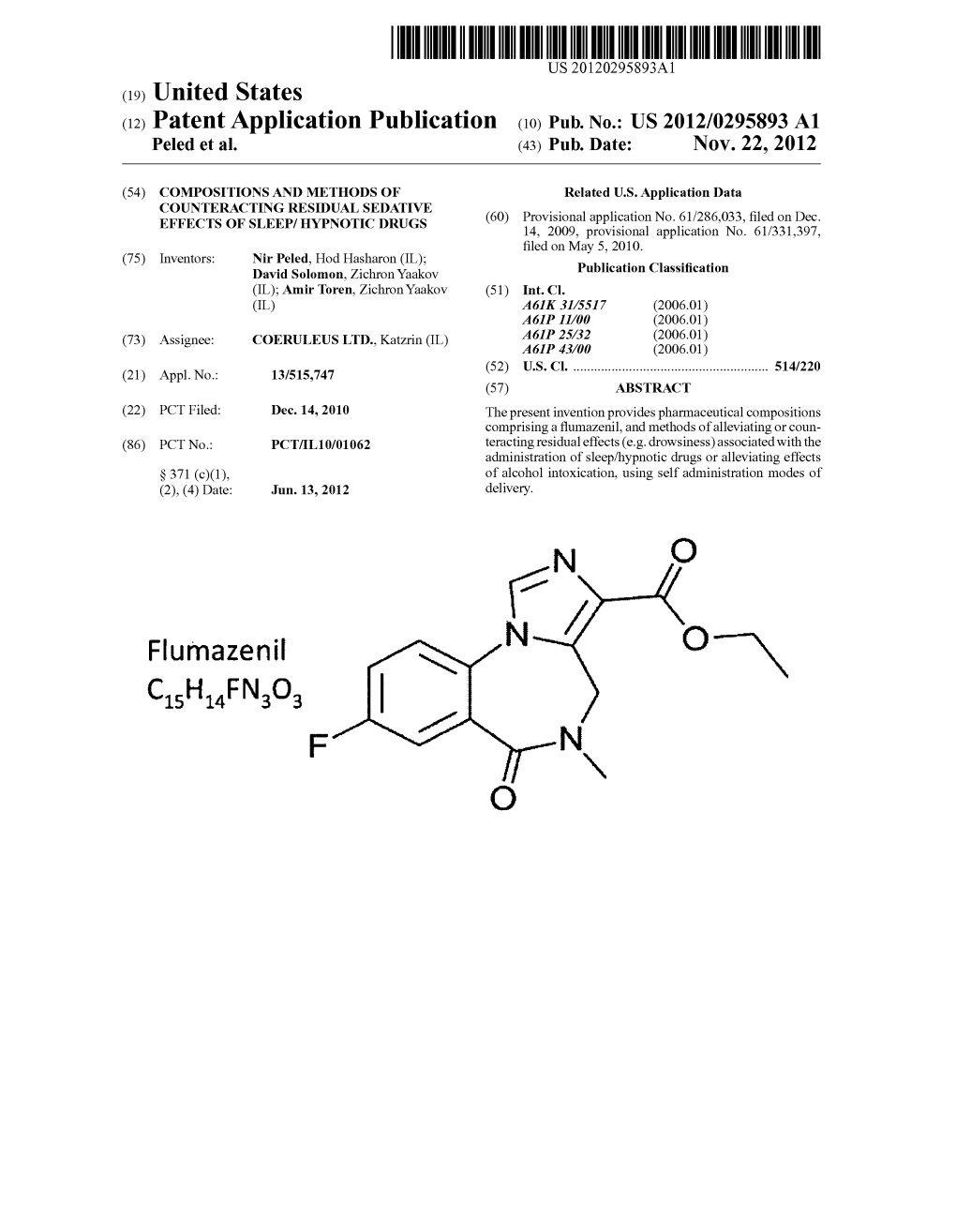
Flumazenil N O—N
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

By ANDREA PETERSEN Getty Images for Those Who Have Trouble
By ANDREA PETERSEN Getty Images For those who have trouble sleeping, there may soon be new ways to summon the sandman. Several pharmaceutical companies are working on new approaches to treat insomnia. The compounds are meant to work differently than current leading sleep aids such as Ambien and Lunesta, which, while generally safe, can have troubling side effects because they act on many areas of the brain. By contrast, many of the drugs being developed target particular systems responsible for sleep and wakefulness. The hope is that they will have fewer side effects and less potential for addiction and cognition problems the next day. New drugs are in the works to treat insomnia, which affects 10% to 30% of Americans (and more women than men). Andrea Peterson explains. About 30% of American adults have insomnia symptoms each year, scientific studies estimate. Some 10% of the population has chronic insomnia, which is generally defined as having difficulty sleeping at least three times a week for a month or more. Chronic insomnia sufferers also feel tired, cranky or foggy-headed during the day. Insomnia comes in various forms. Some people have a tough time falling asleep and others wake in the middle of the night and have trouble getting back to sleep. Some people rise for the day too early. Insomnia can increase the risk for other conditions, including heart disease, diabetes and depression. Merck & Co. is investigating a compound that inhibits the action of orexin receptors, which in turn interferes with the activity of orexin, a chemical in the brain that produces alertness. -

BMC Pharmacology Biomed Central
CORE Metadata, citation and similar papers at core.ac.uk Provided by Springer - Publisher Connector BMC Pharmacology BioMed Central Research article Open Access Pharmacological Properties of DOV 315,090, an ocinaplon metabolite Dmytro Berezhnoy†1, Maria C Gravielle†1, Scott Downing1, Emmanuel Kostakis1, Anthony S Basile2, Phil Skolnick2, Terrell T Gibbs1 and David H Farb*1 Address: 1Laboratory of Molecular Neurobiology, Department of Pharmacology & Experimental Therapeutics, Boston University School of Medicine, 715 Albany St., Boston, MA 02118, USA and 2DOV Pharmaceutical, Inc, 150 Pierce St., Somerset, NJ 08873-4185, USA Email: Dmytro Berezhnoy - [email protected]; Maria C Gravielle - [email protected]; Scott Downing - [email protected]; Emmanuel Kostakis - [email protected]; Anthony S Basile - [email protected]; Phil Skolnick - [email protected]; Terrell T Gibbs - [email protected]; David H Farb* - [email protected] * Corresponding author †Equal contributors Published: 13 June 2008 Received: 20 December 2007 Accepted: 13 June 2008 BMC Pharmacology 2008, 8:11 doi:10.1186/1471-2210-8-11 This article is available from: http://www.biomedcentral.com/1471-2210/8/11 © 2008 Berezhnoy et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Background: Compounds targeting the benzodiazepine binding site of the GABAA-R are widely prescribed for the treatment of anxiety disorders, epilepsy, and insomnia as well as for pre- anesthetic sedation and muscle relaxation. It has been hypothesized that these various pharmacological effects are mediated by different GABAA-R subtypes. -

Preliminary Effects of Pagoclone, a Partial GABA Agonist, On
ORIGINAL RESEARCH Preliminary effects of pagoclone, a partial GABAA agonist, on neuropsychological performance 1 Angela F Caveney Abstract: Pagoclone is a novel cyclopyrrolone that acts as a partial GABAA receptor agonist. Bruno Giordani1 Preclinical studies suggest that pagoclone may have clinical utility as an anxiolytic agent, as George M Haig2 well as a reduced incidence of side-effects. The present study was conducted to determine whether pagoclone would affect healthy individuals’ performances on neuropsychological 1Department of Psychiatry, University of Michigan, Ann Arbor, MI, USA; measures as a function of dose within the projected therapeutic range. Twelve healthy adult 2Neurosciences Development, Abbott subjects were randomly assigned to dosage groups in a 3-way crossover study. Participants Laboratories, Abbott Park, IL, USA were administered neuropsychological measures six hours following dosing on Day 1 and Day 6 of administration of the drug. Dose effects were noted on measures of alertness, learning, and memory and movement time. Signifi cant effects were also noted on measures of alertness, learning and memory, information processing and psychomotor speed. Overall, the results of this small, preliminary study do not support a fi nding of behavioral toxicity for these doses of pagoclone. Rather, a pattern was found of transient and mild negative effects on learning and memory scores at the highest dose administered, though these changes were small and no longer evident by the sixth day of use. Keywords: pagoclone, cyclopyrrolone, neuropsychological, memory, generalized anxiety disorder Introduction Behavioral toxicity refers to the extent to which a drug affects an individual’s ability to perform the psychomotor and cognitive tasks of everyday life (Ramaekers 1998). -

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

GABA Receptors
D Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews Review No.7 / 1-2011 GABA receptors Wolfgang Froestl , CNS & Chemistry Expert, AC Immune SA, PSE Building B - EPFL, CH-1015 Lausanne, Phone: +41 21 693 91 43, FAX: +41 21 693 91 20, E-mail: [email protected] GABA Activation of the GABA A receptor leads to an influx of chloride GABA ( -aminobutyric acid; Figure 1) is the most important and ions and to a hyperpolarization of the membrane. 16 subunits with γ most abundant inhibitory neurotransmitter in the mammalian molecular weights between 50 and 65 kD have been identified brain 1,2 , where it was first discovered in 1950 3-5 . It is a small achiral so far, 6 subunits, 3 subunits, 3 subunits, and the , , α β γ δ ε θ molecule with molecular weight of 103 g/mol and high water solu - and subunits 8,9 . π bility. At 25°C one gram of water can dissolve 1.3 grams of GABA. 2 Such a hydrophilic molecule (log P = -2.13, PSA = 63.3 Å ) cannot In the meantime all GABA A receptor binding sites have been eluci - cross the blood brain barrier. It is produced in the brain by decarb- dated in great detail. The GABA site is located at the interface oxylation of L-glutamic acid by the enzyme glutamic acid decarb- between and subunits. Benzodiazepines interact with subunit α β oxylase (GAD, EC 4.1.1.15). It is a neutral amino acid with pK = combinations ( ) ( ) , which is the most abundant combi - 1 α1 2 β2 2 γ2 4.23 and pK = 10.43. -

Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix
United States International Trade Commission Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix USITC Publication 4208 December 2010 U.S. International Trade Commission COMMISSIONERS Deanna Tanner Okun, Chairman Irving A. Williamson, Vice Chairman Charlotte R. Lane Daniel R. Pearson Shara L. Aranoff Dean A. Pinkert Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix Publication 4208 December 2010 (This page is intentionally blank) Pursuant to the letter of request from the United States Trade Representative of December 15, 2010, set forth at the end of this publication, and pursuant to section 1207(a) of the Omnibus Trade and Competitiveness Act, the United States International Trade Commission is publishing the following modifications to the Harmonized Tariff Schedule of the United States (HTS) to implement changes to the Pharmaceutical Appendix, effective on January 1, 2011. Table 1 International Nonproprietary Name (INN) products proposed for addition to the Pharmaceutical Appendix to the Harmonized Tariff Schedule INN CAS Number Abagovomab 792921-10-9 Aclidinium Bromide 320345-99-1 Aderbasib 791828-58-5 Adipiplon 840486-93-3 Adoprazine 222551-17-9 Afimoxifene 68392-35-8 Aflibercept 862111-32-8 Agatolimod -

204569Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 204569Orig1s000 MEDICAL REVIEW(S) Cross Discipline Team Leader Review 3. CMC/Device Dr. Khairuzzaman found the drug product portion of the NDA to be acceptable, and without need for phase 4 commitments. Dr. Sapru’s review stated that with the exception of a pending issue concerning the control of potential genotoxic impurity (b) (4) the NDA was approvable in terms of drug substance. Dr. Suarez found that the NDA was acceptable from a biopharmaceutics perspective. The Office of Compliance issuance of an acceptable recommendation for drug substance manufacturing and testing facilities was pending at the time of this review. 4. Nonclinical Pharmacology/Toxicology Dr. Richard Siarey completed the primary nonclinical review, and Dr. Lois Freed completed a supervisory memo. Dr. Siarey’s overall conclusion was that from a nonclinical perspective, approval of the suvorexant NDA was recommended. However, he found evidence that catapelxy was observed in dogs exposed to MK-4305 (suvorexant) near Tmax, although he concluded that additional information could have been gained by studying the drug in an experimental model that has been used for diagnosing cataplexy in dogs. Dr. Siarey suggested that since cataplexy occurred in dogs near Tmax, a time at which if used for insomnia patients would ordinarily be in bed, safety concern for humans was reduced. Dr. Siarey also found that the neurobehavioral assessment in the pre- and post-natal developmental study was not complete, as the passive avoidance tests was performed too early in development, while learning/acquisition tests and retention/memory tests were not conducted. -

(12) United States Patent (10) Patent No.: US 8.598,119 B2 Mates Et Al
US008598119B2 (12) United States Patent (10) Patent No.: US 8.598,119 B2 Mates et al. (45) Date of Patent: Dec. 3, 2013 (54) METHODS AND COMPOSITIONS FOR AOIN 43/00 (2006.01) SLEEP DSORDERS AND OTHER AOIN 43/46 (2006.01) DSORDERS AOIN 43/62 (2006.01) AOIN 43/58 (2006.01) (75) Inventors: Sharon Mates, New York, NY (US); AOIN 43/60 (2006.01) Allen Fienberg, New York, NY (US); (52) U.S. Cl. Lawrence Wennogle, New York, NY USPC .......... 514/114: 514/171; 514/217: 514/220; (US) 514/229.5: 514/250 (58) Field of Classification Search (73) Assignee: Intra-Cellular Therapies, Inc. NY (US) None See application file for complete search history. (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 (56) References Cited U.S.C. 154(b) by 215 days. U.S. PATENT DOCUMENTS (21) Appl. No.: 12/994,560 6,552,017 B1 4/2003 Robichaud et al. 2007/0203120 A1 8, 2007 McDevitt et al. (22) PCT Filed: May 27, 2009 FOREIGN PATENT DOCUMENTS (86). PCT No.: PCT/US2O09/OO3261 S371 (c)(1), WO WOOOf77OO2 * 6, 2000 (2), (4) Date: Nov. 24, 2010 OTHER PUBLICATIONS (87) PCT Pub. No.: WO2009/145900 Rye (Sleep Disorders and Parkinson's Disease, 2000, accessed online http://www.waparkinsons.org/edu research/articles/Sleep PCT Pub. Date: Dec. 3, 2009 Disorders.html), 2 pages.* Alvir et al. Clozapine-Induced Agranulocytosis. The New England (65) Prior Publication Data Journal of Medicine, 1993, vol. 329, No. 3, pp. 162-167.* US 2011/0071080 A1 Mar. -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Twelve Cases of Drug-Induced Blepharospasm Improved Within 2 Months of Psychotropic Cessation
Drug, Healthcare and Patient Safety Dovepress open access to scientific and medical research Open Access Full Text Article CASE SERIES Twelve cases of drug-induced blepharospasm improved within 2 months of psychotropic cessation Yuko Emoto1 Background: To determine whether psychotropic cessation in patients with drug-induced Hirofumi Emoto2 blepharospasm improves motor symptoms. Eriko Oishi1 Methods: In patients with drug-induced blepharospasm, we withdrew part or all of their psy- Syunichi Hikita1 chotropic medication and assessed motor symptoms using the Jankovic rating scale (0 = none, Masato Wakakura1 1 = noticeable, 2 = mild, 3 = moderate, 4 = severe) at first presentation and after cessation. Results: Twelve patients (eleven women and one man, mean age 60.4 years) were enrolled. 1Division of Neuro-Ophthalmology, Psychotropics were administered before the onset of blepharospasm in all patients. The mean Inouye Eye Hospital, Tokyo; 2Department of Ophthalmology and duration of treatment with psychotropic medication was 47.3 (range 3–120) months. Jankovic Visual Science, Tokyo Medical and rating scale at initial presentation was 3 in eleven patients and 2 in one patient. After cessation, Dental University, Graduate School of Medicine, Tokyo, Japan blepharospasm started to improve in all cases within 2 months (average 3.9 weeks). While the effect of psychotropic cessation was variable, the symptoms eventually improved to more than 2 on the rating scale. Three of the twelve patients underwent a single botulinum neurotoxin injection and were withdrawn from therapy after cessation. Conclusion: Psychotropic drugs can cause blepharospasm in some cases. Clinicians should consider reducing psychotropic medication as far as possible in patients with blepharospasm taking these agents. -

WO 2015/072852 Al 21 May 2015 (21.05.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/072852 Al 21 May 2015 (21.05.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 36/84 (2006.01) A61K 31/5513 (2006.01) kind of national protection available): AE, AG, AL, AM, A61K 31/045 (2006.01) A61P 31/22 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 31/522 (2006.01) A61K 45/06 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/NL20 14/050780 KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (22) International Filing Date: MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, 13 November 2014 (13.1 1.2014) PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (25) Filing Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 61/903,430 13 November 2013 (13. 11.2013) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: RJG DEVELOPMENTS B.V. -

ETIZOLAM Critical Review Report Agenda Item 4.13
ETIZOLAM Critical Review Report Agenda Item 4.13 Expert Committee on Drug Dependence Thirty-ninth Meeting Geneva, 6-10 November 2017 39th ECDD (2017) Agenda item 4.13 Etizolam Page 2 of 20 39th ECDD (2017) Agenda item 4.13 Etizolam Contents Acknowledgements.................................................................................................................................. 5 Summary...................................................................................................................................................... 6 1. Substance identification ....................................................................................................................... 7 A. International Nonproprietary Name (INN).......................................................................................................... 7 B. Chemical Abstract Service (CAS) Registry Number .......................................................................................... 7 C. Other Chemical Names ................................................................................................................................................... 7 D. Trade Names ....................................................................................................................................................................... 7 E. Street Names ....................................................................................................................................................................... 8 F. Physical Appearance