Projected Second Edition Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Distribution of Tick-Borne Diseases in China Xian-Bo Wu1, Ren-Hua Na2, Shan-Shan Wei2, Jin-Song Zhu3 and Hong-Juan Peng2*
Wu et al. Parasites & Vectors 2013, 6:119 http://www.parasitesandvectors.com/content/6/1/119 REVIEW Open Access Distribution of tick-borne diseases in China Xian-Bo Wu1, Ren-Hua Na2, Shan-Shan Wei2, Jin-Song Zhu3 and Hong-Juan Peng2* Abstract As an important contributor to vector-borne diseases in China, in recent years, tick-borne diseases have attracted much attention because of their increasing incidence and consequent significant harm to livestock and human health. The most commonly observed human tick-borne diseases in China include Lyme borreliosis (known as Lyme disease in China), tick-borne encephalitis (known as Forest encephalitis in China), Crimean-Congo hemorrhagic fever (known as Xinjiang hemorrhagic fever in China), Q-fever, tularemia and North-Asia tick-borne spotted fever. In recent years, some emerging tick-borne diseases, such as human monocytic ehrlichiosis, human granulocytic anaplasmosis, and a novel bunyavirus infection, have been reported frequently in China. Other tick-borne diseases that are not as frequently reported in China include Colorado fever, oriental spotted fever and piroplasmosis. Detailed information regarding the history, characteristics, and current epidemic status of these human tick-borne diseases in China will be reviewed in this paper. It is clear that greater efforts in government management and research are required for the prevention, control, diagnosis, and treatment of tick-borne diseases, as well as for the control of ticks, in order to decrease the tick-borne disease burden in China. Keywords: Ticks, Tick-borne diseases, Epidemic, China Review (Table 1) [2,4]. Continuous reports of emerging tick-borne Ticks can carry and transmit viruses, bacteria, rickettsia, disease cases in Shandong, Henan, Hebei, Anhui, and spirochetes, protozoans, Chlamydia, Mycoplasma,Bartonia other provinces demonstrate the rise of these diseases bodies, and nematodes [1,2]. -

Recognizing and Treating New and Emerging Infections Encountered in Everyday Practice
Recognizing and treating new and emerging infections encountered in everyday practice STEVEN M. GORDON, MD NFECTIOUS DISEASES, pre- MiikWirj:« Although infectious diseases were once considered a dicted earlier in this cen- diminishing threat, new pathogens are constantly challenging tury to be eliminated as a the health care system. This article reviews the clinical presen- public health problem, re- tation, diagnosis, and treatment of seven emerging infections I main the chief cause of death that primary care physicians are likely to encounter. worldwide and a significant cause of death and morbidity in i Parvovirus B19 attacks erythrocyte precursors; the United States.1 Challenging infection is usually benign and self-limiting but can cause the US public health system are aplastic crises in patients with chronic hemolytic disorders. several newly identified patho- Hemorrhagic colitis due to Escherichia coli 0157:H7 infection gens (eg, human immunodefi- can lead to the hemolytic-uremic syndrome, especially in chil- ciency virus [HIV], Escherichia dren; it also can cause thrombotic thrombocytopenia purpura. coli 0157:H7, hepatitis C) and a Chlamydia pneumoniae causes a mild pneumonia that resem- resurgence of old diseases pre- bles mycoplasmal pneumonia. Bacillary angiomatosis primar- sumed to be under control (eg, ily affects immunocompromised patients, especially those tuberculosis, syphilis). Further, infected with human immunodeficiency virus (HIV). At least multiple-drug resistance in two organisms can cause bacillary angiomatosis: Bartonella hense- strains of pneumococci, gono- lae and Bartonella quintana. Hantavirus pulmonary syndrome cocci, enterococci, staphylo- is spread by exposure to the droppings of infected rodents. cocci, salmonella, and mycobac- Contrary to previous thought, HIV continues to replicate teria undermines efforts to throughout the course of the illness and does not have a latency control the diseases they cause.2 phase. -

Q Fever in Small Ruminants and Its Public Health Importance
Journal of Dairy & Veterinary Sciences ISSN: 2573-2196 Review Article Dairy and Vet Sci J Volume 9 Issue 1 - January 2019 Copyright © All rights are reserved by Tolera Tagesu Tucho DOI: 10.19080/JDVS.2019.09.555752 Q Fever in Small Ruminants and its Public Health Importance Tolera Tagesu* School of Veterinary Medicine, Jimma University, Ethiopia Submission: December 01, 2018; Published: January 11, 2019 *Corresponding author: Tolera Tagesu Tucho, School of Veterinary Medicine, Jimma University, Jimma Oromia, Ethiopia Abstract Query fever is caused by Coxiella burnetii, it’s a worldwide zoonotic infectious disease where domestic small ruminants are the main reservoirs for human infections. Coxiella burnetii, is a Gram-negative obligate intracellular bacterium, adapted to thrive within the phagolysosome of the phagocyte. Humans become infected primarily by inhaling aerosols that are contaminated with C. burnetii. Ingestion (particularly drinking raw milk) and person-to-person transmission are minor routes. Animals shed the bacterium in urine and feces, and in very high concentrations in birth by-products. The bacterium persists in the environment in a resistant spore-like form which may become airborne and transported long distances by the wind. It is considered primarily as occupational disease of workers in close contact with farm animals or processing their be commenced immediately whenever Q fever is suspected. To prevent both the introduction and spread of Q fever infection, preventive measures shouldproducts, be however,implemented it may including occur also immunization in persons without with currently direct contact. available Doxycycline vaccines drugof domestic is the first small line ruminant of treatment animals for Q and fever. -

(Scrub Typhus). Incubation Period 1 to 3
TYPHUS Causative Agents TYPHUS Rickettsia typhi (murine typhus) and Orientia tsutsugamushi (scrub typhus). Causative Agents IncubationRickettsia typhi Period (murine typhus) and Orientia tsutsugamushi (scrub typhus). 1 to 3 weeks Incubation Period Infectious1 to 3 weeks Period Zoonoses with no human-to-human transmission. Infectious Period TransmissionZoonoses with no human-to-human transmission. Scrub typhus: Bite of grass mites (larval trombiculid mites) MurineTransmission typhus: Bite of rat fleas (also cat and mice fleas) RodentsScrub typhus: are the Bite preferred of grass and mites normal (larval hosts. trombiculid mites) Murine typhus: Bite of rat fleas (also cat and mice fleas) EpidemiologyRodents are the preferred and normal hosts. Distributed throughout the Asia-Pacific rim and is a common cause of pyrexia of unknownEpidemiology origin throughout SE Asia. Occupational contact with rats (e.g. construDistributedction throughout workers inthe makeAsia-Pshiftacific container rim and isfacilities, a common shop cause owners, of pyrexia granary of workers,unknown andorigin garbage throughout collectors) SE orAsia. exposure Occupational to mite habitat contacts in lonwithg grassrats (e.g. hikersconstru andction so ldiers)workers are inrisk make factors.-shift container facilities, shop owners, granary workers, and garbage collectors) or exposure to mite habitats in long grass (e.g. Inhikers Singapore, and soldiers) a total are ofrisk 13 factors. laboratory confirmed cases of murine typhus were r eported in 2008. The majority of cases were foreign workers. In Singapore, a total of 13 laboratory confirmed cases of murine typhus were Clinicalreported Featuresin 2008. The majority of cases were foreign workers. Fever Clinical Headache Features (prominent) MyalgiaFever ConjunctiHeadache val(prominent) suffusion MaculopapularMyalgia rash Conjunctival suffusion Scrub Maculopapular typhus may alsorash have: relative bradycardia, eschar (80%), painful regional adenopathy, hepatosplenomegaly, meningoencephalitis and renal failure. -

Genetic Diversity of Bartonella Species in Small Mammals in the Qaidam
www.nature.com/scientificreports OPEN Genetic diversity of Bartonella species in small mammals in the Qaidam Basin, western China Huaxiang Rao1, Shoujiang Li3, Liang Lu4, Rong Wang3, Xiuping Song4, Kai Sun5, Yan Shi3, Dongmei Li4* & Juan Yu2* Investigation of the prevalence and diversity of Bartonella infections in small mammals in the Qaidam Basin, western China, could provide a scientifc basis for the control and prevention of Bartonella infections in humans. Accordingly, in this study, small mammals were captured using snap traps in Wulan County and Ge’ermu City, Qaidam Basin, China. Spleen and brain tissues were collected and cultured to isolate Bartonella strains. The suspected positive colonies were detected with polymerase chain reaction amplifcation and sequencing of gltA, ftsZ, RNA polymerase beta subunit (rpoB) and ribC genes. Among 101 small mammals, 39 were positive for Bartonella, with the infection rate of 38.61%. The infection rate in diferent tissues (spleens and brains) (χ2 = 0.112, P = 0.738) and gender (χ2 = 1.927, P = 0.165) of small mammals did not have statistical diference, but that in diferent habitats had statistical diference (χ2 = 10.361, P = 0.016). Through genetic evolution analysis, 40 Bartonella strains were identifed (two diferent Bartonella species were detected in one small mammal), including B. grahamii (30), B. jaculi (3), B. krasnovii (3) and Candidatus B. gerbillinarum (4), which showed rodent-specifc characteristics. B. grahamii was the dominant epidemic strain (accounted for 75.0%). Furthermore, phylogenetic analysis showed that B. grahamii in the Qaidam Basin, might be close to the strains isolated from Japan and China. -

2012 Case Definitions Infectious Disease
Arizona Department of Health Services Case Definitions for Reportable Communicable Morbidities 2012 TABLE OF CONTENTS Definition of Terms Used in Case Classification .......................................................................................................... 6 Definition of Bi-national Case ............................................................................................................................................. 7 ------------------------------------------------------------------------------------------------------- ............................................... 7 AMEBIASIS ............................................................................................................................................................................. 8 ANTHRAX (β) ......................................................................................................................................................................... 9 ASEPTIC MENINGITIS (viral) ......................................................................................................................................... 11 BASIDIOBOLOMYCOSIS ................................................................................................................................................. 12 BOTULISM, FOODBORNE (β) ....................................................................................................................................... 13 BOTULISM, INFANT (β) ................................................................................................................................................... -

Bioterrorism Diseases Annex Infectious Disease Emergency Response (IDER) Plan
Bioterrorism Diseases Annex Infectious Disease Emergency Response (IDER) Plan Contents I Background IV Activation & Notification II Response Organization V Operational Guidance III Purpose & Objectives VI Resources I. BACKGROUND A bioterrorism event is defined for the purposes of this annex as the deliberate introduction of pathogenic microorganisms or their products (bacteria, viruses, fungi or toxins) into a community. Potential bioterrorism agents are categorized by the Centers for Disease Control and Prevention (CDC) by category. Category A agents (highest priority) include organisms that pose a risk to national security because they can be easily disseminated or transmitted from person-to-person; result in high mortality rates and have the potential for major public health impact; might cause public panic and social disruption; and require special action for public health preparedness. These include: • Anthrax (Bacillus anthracis) • Smallpox (variola major) • Botulism (Clostridium botulinum • Tularemia (Franciscella tularensis) toxin) • Viral Hemorrhagic Fevers (filoviruses, • Plague (Yersinia pestis) arenaviruses) Of second highest priority are category B agents which are organisms that are moderately easy to disseminate; that result in moderate morbidity rates and low mortality rates; and that require enhanced diagnostic capacity and disease surveillance. • Brucellosis (Brucella species)* • Epsilon toxin of Clostridium perfringens • Food safety threats (Salmonella species, Escherichia coli O157:H7, Shigella) • Glanders (Burkholderia -

CD Alert Monthly Newsletter of National Centre for Disease Control, Directorate General of Health Services, Government of India
CD Alert Monthly Newsletter of National Centre for Disease Control, Directorate General of Health Services, Government of India May - July 2009 Vol. 13 : No. 1 SCRUB TYPHUS & OTHER RICKETTSIOSES it lacks lipopolysaccharide and peptidoglycan RICKETTSIAL DISEASES and does not have an outer slime layer. It is These are the diseases caused by rickettsiae endowed with a major surface protein (56kDa) which are small, gram negative bacilli adapted and some minor surface protein (110, 80, 46, to obligate intracellular parasitism, and 43, 39, 35, 25 and 25kDa). There are transmitted by arthropod vectors. These considerable differences in virulence and organisms are primarily parasites of arthropods antigen composition among individual strains such as lice, fleas, ticks and mites, in which of O.tsutsugamushi. O.tsutsugamushi has they are found in the alimentary canal. In many serotypes (Karp, Gillian, Kato and vertebrates, including humans, they infect the Kawazaki). vascular endothelium and reticuloendothelial GLOBAL SCENARIO cells. Commonly known rickettsial disease is Scrub Typhus. Geographic distribution of the disease occurs within an area of about 13 million km2 including- The family Rickettsiaeceae currently comprises Afghanistan and Pakistan to the west; Russia of three genera – Rickettsia, Orientia and to the north; Korea and Japan to the northeast; Ehrlichia which appear to have descended Indonesia, Papua New Guinea, and northern from a common ancestor. Former members Australia to the south; and some smaller of the family, Coxiella burnetii, which causes islands in the western Pacific. It was Q fever and Rochalimaea quintana causing first observed in Japan where it was found to trench fever have been excluded because the be transmitted by mites. -

Plague (Yersinia Pestis)
Division of Disease Control What Do I Need To Know? Plague (Yersinia pestis) What is plague? Plague is an infectious disease of animals and humans caused by the bacterium Yersinia pestis. Y. pestis is found in rodents and their fleas in many areas around the world. There are three types of plague: bubonic plague, septicemic plague and pneumonic plague. Who is at risk for plague? All ages may be at risk for plague. People usually get plague from being bitten by infected rodent fleas or by handling the tissue of infected animals. What are the symptoms of plague? Bubonic plague: Sudden onset of fever, headache, chills, and weakness and one or more swollen and painful lymph nodes (called buboes) typically at the site where the bacteria entered the body. This form usually results from the bite of an infected flea. Septicemic plague: Fever, chills, extreme weakness, abdominal pain, shock, and possibly bleeding into the skin and other organs. Skin and other tissues, especially on fingers, toes, and the nose, may turn black and die. This form usually results from the bites of infected fleas or from handling an infected animal. Pneumonic plague: Fever, headache, weakness, and a rapidly developing pneumonia with shortness of breath, chest pain, cough, and sometimes bloody or watery mucous. Pneumonic plague may develop from inhaling infectious droplets or may develop from untreated bubonic or septicemic plague after the bacteria spread to the lungs. How soon do symptoms appear? Symptoms of bubonic plague usually occur two to eight days after exposure, while symptoms for pneumonic plague can occur one to six days following exposure. -
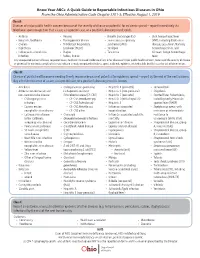
Know Your Abcs: a Quick Guide to Reportable Infectious Diseases in Ohio
Know Your ABCs: A Quick Guide to Reportable Infectious Diseases in Ohio From the Ohio Administrative Code Chapter 3701-3; Effective August 1, 2019 Class A: Diseases of major public health concern because of the severity of disease or potential for epidemic spread – report immediately via telephone upon recognition that a case, a suspected case, or a positive laboratory result exists. • Anthrax • Measles • Rubella (not congenital) • Viral hemorrhagic fever • Botulism, foodborne • Meningococcal disease • Severe acute respiratory (VHF), including Ebola virus • Cholera • Middle East Respiratory syndrome (SARS) disease, Lassa fever, Marburg • Diphtheria Syndrome (MERS) • Smallpox hemorrhagic fever, and • Influenza A – novel virus • Plague • Tularemia Crimean-Congo hemorrhagic infection • Rabies, human fever Any unexpected pattern of cases, suspected cases, deaths or increased incidence of any other disease of major public health concern, because of the severity of disease or potential for epidemic spread, which may indicate a newly recognized infectious agent, outbreak, epidemic, related public health hazard or act of bioterrorism. Class B: Disease of public health concern needing timely response because of potential for epidemic spread – report by the end of the next business day after the existence of a case, a suspected case, or a positive laboratory result is known. • Amebiasis • Carbapenemase-producing • Hepatitis B (perinatal) • Salmonellosis • Arboviral neuroinvasive and carbapenem-resistant • Hepatitis C (non-perinatal) • Shigellosis -

CASE REPORT the PATIENT 33-Year-Old Woman
CASE REPORT THE PATIENT 33-year-old woman SIGNS & SYMPTOMS – 6-day history of fever Katherine Lazet, DO; – Groin pain and swelling Stephanie Rutterbush, MD – Recent hiking trip in St. Vincent Ascension Colorado Health, Evansville, Ind (Dr. Lazet); Munson Healthcare Ostego Memorial Hospital, Lewiston, Mich (Dr. Rutterbush) [email protected] The authors reported no potential conflict of interest THE CASE relevant to this article. A 33-year-old Caucasian woman presented to the emergency department with a 6-day his- tory of fever (103°-104°F) and right groin pain and swelling. Associated symptoms included headache, diarrhea, malaise, weakness, nausea, cough, and anorexia. Upon presentation, she admitted to a recent hike on a bubonic plague–endemic trail in Colorado. Her vital signs were unremarkable, and the physical examination demonstrated normal findings except for tender, erythematous, nonfluctuant right inguinal lymphadenopathy. The patient was admitted for intractable pain and fever and started on intravenous cefoxitin 2 g IV every 8 hours and oral doxycycline 100 mg every 12 hours for pelvic inflammatory disease vs tick- or flea-borne illness. Due to the patient’s recent trip to a plague-infested area, our suspicion for Yersinia pestis infection was high. The patient’s work-up included a nega- tive pregnancy test and urinalysis. A com- FIGURE 1 plete blood count demonstrated a white CT scan from admission blood cell count of 8.6 (4.3-10.5) × 103/UL was revealing with a 3+ left shift and a platelet count of 112 (180-500) × 103/UL. A complete metabolic panel showed hypokalemia and hyponatremia (potassium 2.8 [3.5-5.1] mmol/L and sodium 134 [137-145] mmol/L). -

Select Agents Fact Sheet
SELECT AGENTS FACT SHEET s e ion ecie s n t n p is ms n e e s tio s g s m to a a o u t Rang s p t tme to e th n s n m c a s a ra y a re ho Di P Ge Ho T S Incub F T P Bacteria Bacillus anthracis Humans, cattle, sheep, Direct contact with infected Cutaneous anthrax - skin lesion 2-5 days Fatality rate of 5-20% if Antibiotics: goats, horses, pigs animal tissue, skin, wool developing into a depressed eschar (5- untreated penicillin,ciprofloxacin, hides or their products. 20% case fatality); Inhalation - doxycycline, Inhalation of spores in soil or respiratory distress, fever and shock tetracylines,erythromyci Anthrax hides and wool. Ingestion of with death; Intestinal - abdominal n,chloram-phenicol, contaminated meat. distress followed by fever and neomycin, ampicillin. septicemia Bacteria Brucella (B. Humans, swine, cattle, Skin or mucous membrane High and protracted (extended) fever. 1-15 weeks Most commonly reported Antibiotic combination: melitensis, B. goats, sheep, dogs contact with infected animals, Infection affects bone, heart, laboratory-associated streptomycin, abortus ) their blood, tissue, and other gallbladder, kidney, spleen, and causes bacterial infection in tetracycline, and Brucellosis* body fluids. highly disseminated lesions and man. sulfonamides. abscess Bacteria Yersinia pestis Human; greater than Bite of infected fleas carried Lymphadenitis in nodes with drainage 2-6 days Untreated pneumonic Streptomycin, 200 mammalian species on rodents; airborne droplets from site of flea bite, in lymph nodes and and septicemic plague tetracycline, from humans or pets with inguinal areas, fever, 50% case fatality are fatal; Fleas may chloramphenicol (for plague pneumonia; person-to- untreated; septicemic plague with remain infective for cases of plague Bubonic Plague person transmission by fleas dissemination by blood to meninges; months meningitis), kanamycin secondary pneumonic plague Bacteria Burkholderia mallei Equines, especially Direct contact with nasal 1.