Us Soda Ash in the Late
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

History of the Chlor-Alkali Industry
2 History of the Chlor-Alkali Industry During the last half of the 19th century, chlorine, used almost exclusively in the textile and paper industry, was made [1] by reacting manganese dioxide with hydrochloric acid 100–110◦C MnO2 + 4HCl −−−−−−→ MnCl2 + Cl2 + 2H2O (1) Recycling of manganese improved the overall process economics, and the process became known as the Weldon process [2]. In the 1860s, the Deacon process, which generated chlorine by direct catalytic oxidation of hydrochloric acid with air according to Eq. (2) was developed [3]. ◦ 450–460 C;CuCl2 cat. 4HCl + O2(air) −−−−−−−−−−−−−−→ 2Cl2 + 2H2O(2) The HCl required for reactions (1) and (2) was available from the manufacture of soda ash by the LeBlanc process [4,5]. H2SO4 + 2NaCl → Na2SO4 + 2HCl (3) Na2SO4 + CaCO3 + 2C → Na2CO3 + CaS + 2CO2 (4) Utilization of HCl from reaction (3) eliminated the major water and air pollution problems of the LeBlanc process and allowed the generation of chlorine. By 1900, the Weldon and Deacon processes generated enough chlorine for the production of about 150,000 tons per year of bleaching powder in England alone [6]. An important discovery during this period was the fact that steel is immune to attack by dry chlorine [7]. This permitted the first commercial production and distribu- tion of dry liquid chlorine by Badische Anilin-und-Soda Fabrik (BASF) of Germany in 1888 [8,9]. This technology, using H2SO4 for drying followed by compression of the gas and condensation by cooling, is much the same as is currently practiced. 17 “chap02” — 2005/5/2 — 09Brie:49 — page 17 — #1 18 CHAPTER 2 In the latter part of the 19th century, the Solvay process for caustic soda began to replace the LeBlanc process. -

Solvay Process Company and a Portion of the Village of Solvay Which Grew up Company to Use Their Process
:«:..' :•' Telephone 2-3111 Telephone 2-3111 SYRACUSE JOTJRNAIi Saturday, July 28, 193C Page 8 - SOLVAY PROCESS AMONG STATE'S MIGHTIEST PLANTS MUM) NIK BURNS BRIGHTLY AIRVIEW OF THE SOLVAY PROCESS PLANT WHICH GREW FROM WILLIAM COGSWELL'S IDEA N HISTORY OF VAST IRKS j (This is the fifth of a series of articles whhb willjg <«.»r weklv in the Saturday edition of The Syracuse • ^ourZ^topermitSyracusaJto become iamiliar with the journal, to P"™;*' industrial and commercial enter- inside story of the great industrial a™ * develoo- \ prises which have played important parts in the develop lent of the city.) _ By BICHAKD B. WELCH. Bountiful Nature which supplied Syracuse with huge qnan- Itie* of salt and limestone coupled with the lewdness 0£ a Central New Yorker who saw the l-kto»tta.-* •btainahle raw materials gave Syracuse the.S*j'««- r^™*™™ ATIP of the largest heavy industries in the state. C°T^»M£nk of the history of the Solvay Process r™J»,»T« * subsidiary of the Allied Chemical and Dye Corp- STCE* tSTo William Browne Cog^eUmw, brain tie idea' of utilizing the resources of this section first KenXhdcredit for the formation and progress' »* tfe ^|My industry must also go to Bow and1 Hazard first P^nt tfthe company, and his son, Frederick B. Hazard, who succeeded him. SH; names which burn brightly in the industrial history 01 SMrCCog^ell was born in Oswego, Sept 22,1834 of a lixie- ,ge which dated back to Sir John Cogswell in If5- He was educated in Hamilton Academy at Oneida and in private schools of Syracuse. -

Summer 2017.Pmd
Town of Geddes, 1000 Woods Road, Solvay, NY 13209 www.townofgeddes.com Summer 2017 INSIDE THIS ISSUE: Town of Geddes • Recreation Program and News - Summer Playground Program - Summer Concert Series Summer Concert - Trips and more! • News from the Town Departments Series - Recycling and Garbage Guidelines Concerts are Thursdays from 6:30 p.m. to 8:30 p.m. - Construction Debris & Brush Pickup Rules, etc. July 13 .......... Mario DeSantis Orchestra ...................................Variety At Woods Road Gazebo, Solvay • Senior Citizens Corner Co-Sponsor: Solvay Bank • Community News July 20 .......... Soda Ash Six ...................................................... Dixieland At Cherry Road School, Westvale Co-Sponsor: Lakeland Winery YOUTH LACROSSE CLINIC – July 27 .......... The Rhythm Method ............................................... Rock GIRLS & BOYS AGES 4 TO 8 At Lakeland Park, Lakeland This is an introductory lacrosse program for children ages 4-8 years of age. No Co-Sponsor: Pope’s Grove Golf Course pads and helmets are required. All drills are done with a soft lacrosse ball, so all drills Aug. 3 ........... The Other Guise Band ................................ Oldies/Rock are kid-friendly. Each child must provide his or her own stick. Due to ER Youth At Woods Road Gazebo, Solvay Lacrosse being unable to offer their program this summer, we are offering this program. Aug. 10 ......... Fritz’s Polka Band ................................................Variety Monday through Friday, July 17 to 21 from 6:00 to 7:00 p.m. At Lakeland Park, Lakeland Cherry Road School Field Aug. 17 ......... Flyin’ Column .................................................. Irish, Folk Fee: $25 Geddes residents, $30 nonresidents At Cherry Road School, Westvale Instructors: Camille and Clara O’Kane, Co-Sponsor: Geddes Federal Savings & Loan OCC Lazers Womens’ Lacrosse team members FOR MORE INFORMATION, CALL GEDDES RECREATION DEPT. -

1. Introduction
1. INTRODUCTION Honeywell Intemational Inc. (fonnerly AlliedSignal, Inc.; referred to herein as Honeywell), is conducting aremedial investigation and feasibility study (RI/FS) for Onondaga Lake, which is located near S)'Tacuse, New York (site definition is discussed in Section 1.2 of this RI). The RIfFS is being conducted under the direction of the State ofN ew York and pursuant to the terms of a Consent Decree (Index #89-CV -815) entered into with the State of New York dated January 9, 1992, and associated stipulations (Consent Decree). Onondaga Lake was placed on the USEP A National Priorities List (NFL) (CERCLIS ill number NYD986913580) on December 16, 1994. This NPL listing means that the lake is among the nation's highest priorities for remedial evaluation and responseunder the federal Superfillld law for ~iteswhere there has been a release ofhazardous substances, pollutants, or contaminants. Honeywell submitted a draft RI report in May 1998. Upon review and comment by the US Environmental Protection Agency (USEP A), the New York State Department of Environmental Conservation (NYSDEC), and the New York State Department of Health (NYSDOH), the NYSDEC and the New York State Department of Law (NYSDOL) disapproved this draft document and provided comments to Ho~eywell in August 1999. After completing additional sampling in 1999 and 2000, Honeywell submitted a revised RI report in April 2001 (Exponent, 2001 c). This revised report was similarly disapproved by NYSDEC and NYSDOL in July 2001. Thereafter, pursuant to the Consent Decree, NYSDEC/T AMS Consultants, Inc. (TAMS) prepared this reMite of Honeywell' s revised RI report, with the assistance ofNYSDOH and USEP A. -

Salt In, Salt Out
chapter 2 Salt In, Salt Out Following their organizational meeting in November 1917, Yongli’s promoters began work on two Herculean tasks: searching for a plant design while raising the needed capital. In the process, problems of technology transfer, shifting government policy, and limitations of China’s capital market plagued them. Yongli’s challenge of Brunner, Mond’s formidable hold on the “well regulated” market and technology was both risky and difficult, which in turn made the task of raising the necessary capital even more daunting as it battled the Rev- enue Inspectorate over the gabelle waiver. Problems of Technology Transfer As Chen Diaofu and his colleagues demonstrated in Fan Xudong’s backyard, the chemistry behind the Solvay process was a public good. However, the engineering to make it work on an industrial scale was not.1 Sodium chloride (salt), one of the three main raw materials for the process, must be purified as a solution cleared of dirt, magnesium, and other impurities. The other essential chemical, ammonia, could be generated through burning coke, or added in liquid form. Reaction is then carried out by passing the concentrated and purified brine through the first of two absorption towers. Ammonia bubbles up to saturate the brine (NaCl + NH3, Step I). Separately, carbon dioxide is produced by heating limestone in a kiln at 950–1100°C. The calcium carbonate (CaCO3) in the limestone, the third main ingredient, is partially converted to quicklime (calcium oxide, CaO) and carbon dioxide: CaCO 3 → CO 2 + CaO ( Step II) The carbon dioxide and ammoniacal brine are then fed into a second tower for carbonation. -

OCCASION This Publication Has Been Made Available to the Public on The
OCCASION This publication has been made available to the public on the occasion of the 50th anniversary of the United Nations Industrial Development Organisation. DISCLAIMER This document has been produced without formal United Nations editing. The designations employed and the presentation of the material in this document do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations Industrial Development Organization (UNIDO) concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries, or its economic system or degree of development. Designations such as “developed”, “industrialized” and “developing” are intended for statistical convenience and do not necessarily express a judgment about the stage reached by a particular country or area in the development process. Mention of firm names or commercial products does not constitute an endorsement by UNIDO. FAIR USE POLICY Any part of this publication may be quoted and referenced for educational and research purposes without additional permission from UNIDO. However, those who make use of quoting and referencing this publication are requested to follow the Fair Use Policy of giving due credit to UNIDO. CONTACT Please contact [email protected] for further information concerning UNIDO publications. For more information about UNIDO, please visit us at www.unido.org UNITED NATIONS INDUSTRIAL DEVELOPMENT ORGANIZATION Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria Tel: (+43-1) 26026-0 · www.unido.org · [email protected] k r i RESTRICTED MARKET STUDY F J <"* FOR SEA CHEMICALS PROJECT I N ICELAND PREPARED FOR NATIONAL RESEARCH COUNCIL REYKJAVIK, ICELAND BY WILLIAM B. -

Onondaga Lake Educational Unit
Onondaga Lake Educational Unit Onondaga Lake Educational Unit Supplemental Curriculum Materials for Secondary Teachers and Students in Science, Social Studies, English, and Technology Heidi Busa, John Birmingham, Richard Beal, and KBB Sobering ESF Educational Outreach 129 Bray Hall- Outreach, SUNY ESF 1 Forestry Drive Syracuse, NY 13210 (315) 470-6817 FAX (315) 470-6818 Email: [email protected] Copyright © 2004 SUNY College of Environmental Science and Forestry. All rights reserved. SUNY ESF 1 Onondaga Lake Educational Unit Instructor Overview The Onondaga Lake Educational Unit is a series of lessons designed to investigate issues related to Onondaga Lake. The lessons are interdisciplinary and have been correlated to the New York State Standards for Math, Science and Technology, and Social Studies; the lessons meet the needs of both teachers and students. Collaboration between teachers can show students that the issues overlap many subject areas. The impact of humans on their environment is easy to observe in the case of Onondaga Lake. The health of this important resource is severely compromised and this series of lessons investigates the many variables involved in returning it to a healthier state. The lessons may be presented in any order or used individually to emphasize topics of interest to the class. The Onondaga Lake Educational Unit is intended to illustrate how humans can impact an environment and the issues involved in correcting problems created by this impact. SUNY ESF 2 Onondaga Lake Educational Unit Onondaga Lake Educational Unit Instructor Overview Course Objective: The ESF Environmental Science Educational Units provide high school teachers with student-centered, interdisciplinary lessons that cover the New York State Standards for Math, Science, and Technology, and Social Studies. -
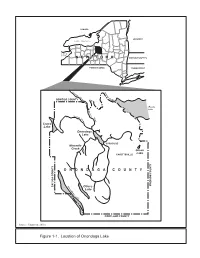
Figure 1-1. Location of Onondaga Lake S Creek E C a R L N E R E Il I V M W Lake Outlet a S
CANADA VERMONT Lake Ontario Lake Erie NEW YORK MASSACHUSETTS PENNSYLVANIA CONNECTICUT Oswego Oneida River OSWEGO COUNTY River Oneida Lake Seneca Seneca River Cross River Cross Lake Lake Onondaga Lake SYRACUSE Ninemile Creek Green Lake FAYETTEVILLE ONONDAGA COUNTY Skaneateles MADISON COUNTY CAYUGA COUNTY CAYUGA Otisco Lake Lake CORTLAND COUNTY Source: Exponent, 2001c Figure 1-1. Location of Onondaga Lake S Creek e c a r ll n e R e i i v m w Lake Outlet a S Liverpool ,'90 k o ro B dy o Mattydale lo Onondaga B Lakeland Lake Galeville k e N e inem r ile C Cr y eek 690 e ,' L Wes t F T lum r e ib k u o ta o r Onondaga Creek r y M B 5 A e s tr e o Unnamed Creek d d East Flume e H G ar bo Syracuse Solvay r B 695 ro ,' o k Fairmount k o o r Westvale B r bo ar H ,'81 LEGEND River or brook River or brook (below grade) Major road Minor road 0 1 2 Miles 0 1 2 3 Kilometers Source: NYSDOT (no date) Modified from Exponent, 2001c Figure 1-2. Onondaga Lake Area Tributaries and Roads LEGEND Creek a Approximate Location Sawmill and Extent Lake Outlet LIVERPOOL Brook Bloody O n o n d a g a L a k e GALEVILLE Dredge Spoils Areaa LAKELAND Creek Ninemile Creek Ley Former Willis Brook In-lake West Avenue Plant Semet Waste SYRACUSE East Deposita Flume Residue Flume Ponds Tributary Onondaga Geddes 5A Creek Metro Former Mathews LCP Bridge Main Plant Brook Ave. -

Consent Order As Appendix A
, STATE OF NEW YORK: DEPARTMENT OF ENVIRONMENTAL CONSERVATION In the Matter of the Alleged Violations of Articles 3, 11, 15, 17, 24, 27 titles 7 and 13, and Article 40 of the Environmental Conservation Law of the State ofNew York, and of Title 6 Official CONSENT Compilation of Codes, Rule and Regulations ORDER of the State ofNew York filedpursuant thereto Index No. D-7-0001-02-03 - by - Site No. 7-34-076 Honeywell International Inc., (Solvay Wastebeds 9-15) Respondent. --c---------------------- WHEREAS, 1. The New York State Department of Environmental Conservation ("the Department") is an executive department of the State of New York with jurisdiction over the environmental policy and laws of this state and has the power to provide forthe prevention and abatement of all water, land, and air pollution under, inter alia, the Environmental Conservation Law ("ECL") §3-0301.1.i. The Depaiiment is also responsible forthe enforcement ofECL articles 3, 11, 15, 17, 24, 27 titles 7 and 13, and aiiicles 40 and 71. 2. Honeywell InternationalInc., formerlyknown, in part, as AlliedSignal Inc. and the Solvay Process Company ("Respondent"), is a corporation organized and existing under the laws of the State of Delaware and is a "person" as defined, inter alia, in ECL §§ I 7-0105(1) and 27-0303(3), and at 6 NYCRR subparts 360-1.2(b)(l 17) and 750-1.2(64). 3. Respondent owns property in New York State located in the Towns of Geddes and Camillus, Onondaga County, and identifiedby Respondent as Wastebeds 9, 10, 11, 12, 13, 14 and 15. -

Translations Whatever Happened to Homberg's
9 h plt prr nldn lt f ttnd vn dtr nrl f lbrr rrh t nrvl th tr n Wrdr "rdn f th Cnr n Chtr ld n h rlt hh t ntrtn ppr th Ch Illn At 1 t 193" . A. Ch. S., 193 Whatever Happened To ... ? ln , 35 (n nbr d Otbr 1h ppr r ttrd thrht . A. Ch. S. fr WHATEVER HAPPENED TO HOMBERG'S 193 nd 19 bt r prl dntfd hvn rntd t th PYROPHORUS? Cnr 11 W Clr t l "Intrntnl Chl Cnr" . William B. Jensen, University of Cincinnati A. Ch. S., 19 6, 880. 1h nl ntn f th rl tn b th St "br rphr" dntll dvrd b rrd n th prlnr nnnnt fr th nd Intrntnl Wlhl br (15-1715 t rnd 1 hl Cnr f Appld Chtr hld n r n 19; Ann . ttptn t extract n "drl ht l" fr hn A. Ch. S., 19 , 37 xrnt fr th prp f trntn rr nt lvr (1 In th r f th xprnt br dtlld th xrnt th d vrt f thr trl n f James J. Bohning is Professor of Chemistry at Wilkes College, hh hppnd t b n pth r pt l Wilkes-Barre, PA 18766 and is particularly interested in the [K2(SO4Al2(SO4321 nd ntd tht ftr ln th history of the ACS. He is a Past-Chair of the Division and is pprt nd brn pn th ltn th dr rd n th currently serving as its historian. rtrt pntnl brt nt fl h rlt t ntrll ht br ttntn n f h bdn fntn l tht f n f h ntprr th th prprtn nd td f tr- TRANSLATIONS l hh r thr pntnl nflbl r phph- rnt r bth Indd drn h tdnt trvl n Itl h The following experiment is taken from Tiberius Cavallo' s "A hd nvttd th prprtn nd prprt f th -lld Treatise on the Nature and Properties of Air," London, 1781. -

027402A0.Pdf
402 NATURE [Feb. 22, 1883 x88o they produced x8,8oo tom, and their output is now at the and at the same time will ccnfer an inestimable boon on the towns rate of 52,000 tons per annum. The new works no•v in course where coal is now largely used as fuel. of construction in this country and on the Continent, when com These three couroes, says Mr. Weldon, must be all adopted by pleted, will at once increase the production of ammonia soda by the English soda-maker. If, in addition to doing thi•, the 65,exx> to 7o,ooo tons annually. strictest economy in manufacture is practised and the purest and What then can the manufacturer of Leblanc soda expect save best product that can be made is always turned out, the manu· utter collapse? But the state of the alkali-maker threatens to facturer of soda by the old Leblanc method may yet hope to become even worse than it is. The source of the sulphur which hold his own against the new and wonderfully successful is used in the Leblanc process is pyrites ; the pyrites employed ammonia proce>s. M. M. P. M. in this country is almost exclusively imported by three large companies from Spain and Portugal ; it contains from 2 to 3 per cent. of copper, and very small quantities of silver and gold. UNIVERSITY AND EDUCATIONAL When the soda manufacturer has burnt off the sulphur, he >ends INTELLIGENCE the residual ore to the -copper extractor, who is able to sell the OxFORD.-The following persons have been elected Members iron oxide which remains when he has taken out the copper at of the Committee for the nomination of examiners in the Natural ab::mt 12s. -

Fugitive Emissions)
AEAT/R/ENV/1000 Issue 1 Appendix 3 Energy (Fugitive Emissions) CONTENTS 1 INTRODUCTION 2 2 COAL MINING 2 3 SOLID FUEL TRANSFORMATION 3 3.1 Coke Production 3 3.2 Solid Smokeless Fuel Production 4 4 OIL AND NATURAL GAS 5 4.1 Offshore Flaring 6 4.2 Offshore Gas Use 7 4.3 Well Testing 8 4.4 Other Emissions from Platforms and Terminals 9 4.5 Loading Emissions 9 4.6 Leakage from the Gas Transmission System. 10 4.7 Petrol Distribution 11 4.8 Refineries and Petroleum Processes 11 4.9 Gasification Processes 12 5 REFERENCES 13 AEA Technology A3.1 AEAT/R/ENV/1000 Issue 1 1 Introduction This Appendix outlines the emissions of greenhouse gases arising from the production, extraction of coal, oil and natural gas; their storage, processing and distribution. These emissions are fugitive emissions and are reported in IPCC Table 1B. Emissions from fuel combustion during these processes are reported in IPCC Table 1A and are described in Appendix 2. In certain cases the methodology of some of these fuel combustion emissions are discussed in this Appendix, because they have links with the methodologies used for fugitive emissions. 2 Coal Mining The NAEI reports emissions of methane from coal mining in the categories deep mined coal; coal storage and transport; open cast coal. These map onto the IPCC categories 1B1ai Underground Mines-mining, 1B1ai Underground Mines-post-mining and 1B1aii Surface Mines respectively. Emissions are calculated from saleable coal production statistics reported in DTI, (2001). Data on the shallower licensed mines are not published and were supplied to us by Barty (1995) up to 1994.