The Effect of Additives and Temperature on the Rheological Behavior of Invert (W/O) Emulsions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bingham Yield Stress and Bingham Plastic Viscosity of Homogeneous Non-Newtonian Slurries
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Cape Peninsula University of Technology BINGHAM YIELD STRESS AND BINGHAM PLASTIC VISCOSITY OF HOMOGENEOUS NON-NEWTONIAN SLURRIES by Brian Tonderai Zengeni Student Number: 210233265 Dissertation submitted in partial fulfilment of the requirements for the degree and which counts towards 50% of the final mark Master of Technology Mechanical Engineering in the Faculty of Engineering at the Cape Peninsula University of Technology Supervisor: Prof. Graeme John Oliver Bellville Campus October 2016 CPUT copyright information The dissertation/thesis may not be published either in part (in scholarly, scientific or technical journals), or as a whole (as a monograph), unless permission has been obtained from the University DECLARATION I, Brian Tonderai Zengeni, declare that the contents of this dissertation represent my own unaided work, and that the dissertation has not previously been submitted for academic examination towards any qualification. Furthermore, it represents my own opinions and not necessarily those of the Cape Peninsula University of Technology. October 2016 Signed Date i ABSTRACT This dissertation presents how material properties (solids densities, particle size distributions, particle shapes and concentration) of gold tailings slurries are related to their rheological parameters, which are yield stress and viscosity. In this particular case Bingham yield stresses and Bingham plastic viscosities. Predictive models were developed from analysing data in a slurry database to predict the Bingham yield stresses and Bingham plastic viscosities from their material properties. The overall goal of this study was to develop a validated set of mathematical models to predict Bingham yield stresses and Bingham plastic viscosities from their material properties. -

Mapping Thixo-Visco-Elasto-Plastic Behavior
Manuscript to appear in Rheologica Acta, Special Issue: Eugene C. Bingham paper centennial anniversary Version: February 7, 2017 Mapping Thixo-Visco-Elasto-Plastic Behavior Randy H. Ewoldt Department of Mechanical Science and Engineering, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA Gareth H. McKinley Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA Abstract A century ago, and more than a decade before the term rheology was formally coined, Bingham introduced the concept of plastic flow above a critical stress to describe steady flow curves observed in English china clay dispersion. However, in many complex fluids and soft solids the manifestation of a yield stress is also accompanied by other complex rheological phenomena such as thixotropy and viscoelastic transient responses, both above and below the critical stress. In this perspective article we discuss efforts to map out the different limiting forms of the general rheological response of such materials by considering higher dimensional extensions of the familiar Pipkin map. Based on transient and nonlinear concepts, the maps first help organize the conditions of canonical flow protocols. These conditions can then be normalized with relevant material properties to form dimensionless groups that define a three-dimensional state space to represent the spectrum of Thixotropic Elastoviscoplastic (TEVP) material responses. *Corresponding authors: [email protected], [email protected] 1 Introduction One hundred years ago, Eugene Bingham published his first paper investigating “the laws of plastic flow”, and noted that “the demarcation of viscous flow from plastic flow has not been sharply made” (Bingham 1916). Many still struggle with unambiguously separating such concepts today, and indeed in many real materials the distinction is not black and white but more grey or gradual in its characteristics. -

Modification of Bingham Plastic Rheological Model for Better Rheological Characterization of Synthetic Based Drilling Mud
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Covenant University Repository Journal of Engineering and Applied Sciences 13 (10): 3573-3581, 2018 ISSN: 1816-949X © Medwell Journals, 2018 Modification of Bingham Plastic Rheological Model for Better Rheological Characterization of Synthetic Based Drilling Mud Anawe Paul Apeye Lucky and Folayan Adewale Johnson Department is Petroleum Engineering, Covenant University, Ota, Nigeria Abstract: The Bingham plastic rheological model has generally been proved by rheologists and researchers not to accurately represent the behaviour of the drilling fluid at very low shear rates in the annulus and at very high shear rate at the bit. Hence, a dimensionless stress correction factor is required to correct these anomalies. Hence, this study is coined with a view to minimizing the errors associated with Bingham plastic model at both high and low shear rate conditions bearing in mind the multiplier effect of this error on frictional pressure loss calculations in the pipes and estimation of Equivalent Circulating Density (ECD) of the fluid under downhole conditions. In an attempt to correct this anomaly, the rheological properties of two synthetic based drilling muds were measured by using an automated Viscometer. A comparative rheological analysis was done by using the Bingham plastic model and the proposed modified Bingham plastic model. Similarly, a model performance analysis of these results with that of power law and Herschel Buckley Model was done by using statistical analysis to measure the deviation of stress values in each of the models. The results clearly showed that the proposed model accurately predicts mud rheology better than the Bingham plastic and the power law models at both high and low shear rates conditions. -

A Review on Recent Developments in Magnetorheological Fluid Based Finishing Processes
© 2018 JETIR June 2018, Volume 5, Issue 6 www.jetir.org (ISSN-2349-5162) A REVIEW ON RECENT DEVELOPMENTS IN MAGNETORHEOLOGICAL FLUID BASED FINISHING PROCESSES Kanthale V. S., Dr. Pande D. W. College of Engineering Pune, India ABSTRACT-The recent engineering applications demand the use of tough, high intensity and high temperature resistant materials in technology, which evolved the need for newer and better finishing techniques. The conventional machining or finishing methods are not workable for the hard materials like carbides; ceramics, die steel. Magnetorheological fluid assisted finishing process is a new technique under development and becoming popular in this epoch. With the use of external magnetic field, the rheological properties of the MR fluid can be controlled for polishing the surfaces of components. An extended survey of the material removal mechanism and subsequent evolution of analytical MR models are necessary for raising the finishing operation of the MR fluid process. Understanding the influence of various process parameters on the process performance measures such as surface finish and material removal rate. The objective of this study is to do a comprehensive, critical and extensive review of various material removal models based on MR fluid in terms of experimental mechanism, finding the effects of process parameters, characterization and rheological properties of MR fluid. This paper deals with various advancements in MR fluid based process and its allied processes have been discussed in the making the developments in the future in these processes. KEYWORDS: Magnetorheological fluid (MR fluid), Process parameter, Surface roughness, Material removal rate 1. INTRODUCTION The modern engineering applications have necessitated high precision manufacturing. -

Rough-Wall and Turbulent Transition Analyses for Bingham Plastics
THOMAS, A.D. and WILSON, K.C. Rough-wall and turbulent transition analyses for Bingham plastics. Hydrotransport 17. The 17th International Conference on the Hydraulic Transport of Solids, The Southern African Institute of Mining and Metallurgy and the BHR Group, 2007. Rough-wall and turbulent transition analyses for Bingham plastics A.D. THOMAS* and K.C. WILSON† *Slurry Systems Pty Ltd, Australia †Queens University, Canada Some years ago, the authors developed an analysis of non- Newtonian pipe flow based on enhanced turbulent microscale and associated sub-layer thickening. This analysis, which can be used for various rheologic modelling equations, has in the past been applied to smooth-walled pipes. The present paper extends the analysis to rough walls, using the popular Bingham plastic equation as a representative rheological behaviour in order to prepare friction-factor plots for comparison with measured results. The conditions for laminar-turbulent transition for both smooth and rough walls are also considered, and comparisons are made with earlier correlations and with experimental data. Notation D internal pipe diameter f friction factor (Darcy-Weisbach) fT value of f at laminar-turbulent transition He Hedström number (see Equation [2]) k representative roughness height Re* shear Reynolds number (DU*/) Re*k Reynolds number based on roughness (kU*/ ) ReB Bingham plastic Reynolds number (VD/B) U local velocity at distance y from wall U* shear velocity (͌[o/]) V mean (flow) velocity VN mean (flow) velocity for a Newtonian fluid -

Non Newtonian Fluids
Non-Newtonian Fluids Non-Newtonian Flow Goals • Describe key differences between a Newtonian and non-Newtonian fluid • Identify examples of Bingham plastics (BP) and power law (PL) fluids • Write basic equations describing shear stress and velocities of non-Newtonian fluids • Calculate frictional losses in a non-Newtonian flow system Non-Newtonian Fluids Newtonian Fluid du t = -µ z rz dr Non-Newtonian Fluid du t = -h z rz dr η is the apparent viscosity and is not constant for non-Newtonian fluids. η - Apparent Viscosity The shear rate dependence of η categorizes non-Newtonian fluids into several types. Power Law Fluids: Ø Pseudoplastic – η (viscosity) decreases as shear rate increases (shear rate thinning) Ø Dilatant – η (viscosity) increases as shear rate increases (shear rate thickening) Bingham Plastics: Ø η depends on a critical shear stress (t0) and then becomes constant Non-Newtonian Fluids Bingham Plastic: sludge, paint, blood, ketchup Pseudoplastic: latex, paper pulp, clay solns. Newtonian Dilatant: quicksand Modeling Power Law Fluids Oswald - de Waele n é n-1 ù æ du z ö æ du z ö æ du z ö t rz = Kç- ÷ = êKç ÷ ú ç- ÷ è dr ø ëê è dr ø ûú è dr ø where: K = flow consistency index n = flow behavior index µeff Note: Most non-Newtonian fluids are pseudoplastic n<1. Modeling Bingham Plastics du t <t z = 0 Rigid rz 0 dr du t ³t t = -µ z ±t rz 0 rz ¥ dr 0 Frictional Losses Non-Newtonian Fluids Recall: L V 2 h = 4 f f D 2 Applies to any type of fluid under any flow conditions Laminar Flow Mechanical Energy Balance Dp DaV 2 + + g Dz + h =Wˆ -
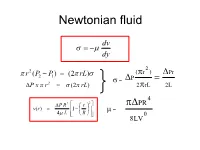
Non-Newtonian Fluids Flow Characteristic of Non-Newtonian Fluid
Newtonian fluid dv dy 2 r 2 (P P ) (2 rL) (r ) Pr 2 1 P 2 = P x r (2 rL) 2 rL 2L 2 4 P R2 r PR v(r) 1 4 L R = 8LV0 Definition of a Newtonian Fluid F du yx yx A dy For Newtonian behaviour (1) is proportional to and a plot passes through the origin; and (2) by definition the constant of proportionality, Newtonian dv dy 2 r 2 (P P ) (2 rL) (r ) Pr 2 1 P 2 = P x r (2 rL) 2 rL 2L 2 4 P R2 r PR v(r) 1 4 L R = 8LV0 Newtonian 2 From (r ) Pr d u = P 2rL 2L d y 4 and PR 8LQ = 0 4 8LV P = R = 8v/D 5 6 Non-Newtonian Fluids Flow Characteristic of Non-Newtonian Fluid • Fluids in which shear stress is not directly proportional to deformation rate are non-Newtonian flow: toothpaste and Lucite paint (Casson Plastic) (Bingham Plastic) Viscosity changes with shear rate. Apparent viscosity (a or ) is always defined by the relationship between shear stress and shear rate. Model Fitting - Shear Stress vs. Shear Rate Summary of Viscosity Models Newtonian n ( n 1) Pseudoplastic K n Dilatant K ( n 1) n Bingham y 1 1 1 1 Casson 2 2 2 2 0 c Herschel-Bulkley n y K or = shear stress, º = shear rate, a or = apparent viscosity m or K or K'= consistency index, n or n'= flow behavior index Herschel-Bulkley model (Herschel and Bulkley , 1926) n du m 0 dy Values of coefficients in Herschel-Bulkley fluid model Fluid m n Typical examples 0 Herschel-Bulkley >0 0<n< >0 Minced fish paste, raisin paste Newtonian >0 1 0 Water,fruit juice, honey, milk, vegetable oil Shear-thinning >0 0<n<1 0 Applesauce, banana puree, orange (pseudoplastic) juice concentrate Shear-thickening >0 1<n< 0 Some types of honey, 40 % raw corn starch solution Bingham Plastic >0 1 >0 Toothpaste, tomato paste Non-Newtonian Fluid Behaviour The flow curve (shear stress vs. -

Bingham Plastic Characteristic of Blood Flow Through a Generalized Atherosclerotic Artery with Multiple Stenoses
Available online at www.pelagiaresearchlibrary.com Pelagia Research Library Advances in Applied Science Research, 2012, 3 (6):3551-3557 ISSN: 0976-8610 CODEN (USA): AASRFC Bingham Plastic characteristic of blood flow through a generalized atherosclerotic artery with multiple stenoses S. S. Yadav and Krishna Kumar Narain (P.G.) College, Shikohabad (India) _____________________________________________________________________________________________ ABSTRACT In this paper, we have investigated the effects of length of stenosis, shape parameter, parameter γ on the resistance to flow for a Bingham plastic flow of blood through a generalized artery having multiple stenoses locate at equal distances. Here, the rheology of the flowing blood is characterized by non-Newtonian fluid model. The study reveals that flow resistance decreases as shape parameter and γ increases and it increases as stenosis height and length increase. We have also shown the variation of wall shear stress for different values of parameter γ . Keywords: Resistance to flow, wall shear stress, stenosis, shape parameter _____________________________________________________________________________________________ INTRODUCTION Hardening of the arteries is a procedure that often occurs with aging. As we grow older, plaque buildup narrows our arteries and makes them stiffer. These changes make it harder for blood to flow through them. Clots may form in these narrowed arteries and block blood flow. Pieces of plaque can also break off and move to smaller blood vessels, blocking them. Either way, the blockage starves tissues of blood and oxygen, which can result in damage or tissue death. This is a common cause of heart attack and stroke. High blood cholesterol levels can cause hardening of the arteries at a younger age. -

The Effect of Thixotropy on Pressure Losses in a Pipe
energies Article The Effect of Thixotropy on Pressure Losses in a Pipe Eric Cayeux * and Amare Leulseged Norwegian Research Centre, 4021 Stavanger, Norway; [email protected] * Correspondence: [email protected]; Tel.: +47-47-501-787 Received: 23 October 2020; Accepted: 20 November 2020; Published: 24 November 2020 Abstract: Drilling fluids are designed to be shear-thinning for limiting pressure losses when subjected to high bulk velocities and yet be sufficiently viscous to transport solid material under low bulk velocity conditions. They also form a gel when left at rest, to keep weighting materials and drill-cuttings in suspension. Because of this design, they also have a thixotropic behavior. As the shear history influences the shear properties of thixotropic fluids, the pressure losses experienced in a tube, after a change in diameter, are influenced over a much longer distance than just what would be expected from solely entrance effects. In this paper, we consider several rheological behaviors that are relevant for characterizing drilling fluids: Collins–Graves, Herschel–Bulkley, Robertson–Stiff, Heinz–Casson, Carreau and Quemada. We develop a generic solution for modelling the viscous pressure gradient in a circular pipe under the influence of thixotropic effects and we apply this model to configurations with change in diameters. It is found that the choice of a rheological behavior should be guided by the actual response of the fluid, especially in a turbulent flow regime, and not chosen a priori. Furthermore, thixotropy may influence pressure gradients over long distances when there are changes of diameter in a hydraulic circuit. This fact is important to consider when designing pipe rheometers. -

Optimal Design Methodology of Magnetorheological Fluid Based Mechanisms
Chapter 14 Optimal Design Methodology of Magnetorheological Fluid Based Mechanisms Quoc-Hung Nguyen and Seung-Bok Choi Additional information is available at the end of the chapter http://dx.doi.org/10.5772/51078 1. Introduction Magnetorheological fluid (MRF) is a non-colloidal suspension of magnetizable particles that are on the order of tens of microns (20-50 microns) in diameter. Generally, MRF is composed of oil, usually mineral or silicone based, and varying percentages of ferrous particles that have been coated with an anti-coagulant material. When inactivated, MRF displays Newtonian-like behavior. When exposed to a magnetic field, the ferrous particles that are dispersed throughout the fluid form magnetic dipoles. These magnetic dipoles align themselves along lines of magnetic flux. The fluid was developed by Jacob Rabinow at the US National Bureau of Standards in the late 1940's. For the first few years, there was a flurry of interest in MRF but this interest quickly waned. In the early 1990's there was resurgence in MRF research that was primarily due to Lord Corporation's research and development. Although similar in operation to electro-rheological fluids (ERF) and Ferro-fluids, MR devices are capable of much higher yield strengths when activated. For this advantage, many MRF-based mechanisms have been developed such as MR dampers, MR brake, MR clutch, MR valve... and some of them are now commercial. As well-known that performance of MRF based systems significantly depends on the activating magnetic circuit, therefore, by optimal design of the activating magnetic circuit, the performance of MRF-based systems can be optimized. -

A Basic Introduction to Rheology
WHITEPAPER A Basic Introduction to Rheology RHEOLOGY AND Introduction VISCOSITY Rheometry refers to the experimental technique used to determine the rheological properties of materials; rheology being defined as the study of the flow and deformation of matter which describes the interrelation between force, deformation and time. The term rheology originates from the Greek words ‘rheo’ translating as ‘flow’ and ‘logia’ meaning ‘the study of’, although as from the definition above, rheology is as much about the deformation of solid-like materials as it is about the flow of liquid-like materials and in particular deals with the behavior of complex viscoelastic materials that show properties of both solids and liquids in response to force, deformation and time. There are a number of rheometric tests that can be performed on a rheometer to determine flow properties and viscoelastic properties of a material and it is often useful to deal with them separately. Hence for the first part of this introduction the focus will be on flow and viscosity and the tests that can be used to measure and describe the flow behavior of both simple and complex fluids. In the second part deformation and viscoelasticity will be discussed. Viscosity There are two basic types of flow, these being shear flow and extensional flow. In shear flow fluid components shear past one another while in extensional flow fluid component flowing away or towards from one other. The most common flow behavior and one that is most easily measured on a rotational rheometer or viscometer is shear flow and this viscosity introduction will focus on this behavior and how to measure it. -

Existence of Weak Solutions for a Bingham Fluid-Rigid Body System Benjamin Obando, Takéo Takahashi
Existence of weak solutions for a Bingham fluid-rigid body system Benjamin Obando, Takéo Takahashi To cite this version: Benjamin Obando, Takéo Takahashi. Existence of weak solutions for a Bingham fluid-rigid body system. Annales de l’Institut Henri Poincaré (C) Non Linear Analysis, Elsevier, 2019, 36 (5), pp.1281- 1309. 10.1016/j.anihpc.2018.12.001. hal-01942426 HAL Id: hal-01942426 https://hal.archives-ouvertes.fr/hal-01942426 Submitted on 3 Dec 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Existence of weak solutions for a Bingham fluid-rigid body system Benjamin Obando1,2 and Tak´eoTakahashi2 1Departamento de Ingenier´ıaMatem´atica,Universidad de Chile, Av. Blanco Encalada 2120, Casilla 170-3, Correo 3, Santiago, Chile 2Universit´ede Lorraine, CNRS, Inria, IECL, F-54000 Nancy November 30, 2018 Contents 1 Introduction and main result 2 2 Notation and preliminary results 6 2.1 Weak form and energy inequality . .7 2.2 Junction of solenoidal fields . .9 3 Approximated Problems 12 4 Passing to the limit M ! 1 and " ! 0 16 4.1 Weak convergences . 16 4.2 Strong convergence of the velocity .