Effect of Enzymes, Hydrocolloids, and Emulsifiers on Qualities of Dough and Bread Made from Whole Grain Wheat Flour
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2020 Annual Recipe SIP.Pdf
SPECIAL COLLECTOR’SEDITION 2020 ANNUAL Every Recipe from a Full Year of America’s Most Trusted Food Magazine CooksIllustrated.com $12.95 U.S. & $14.95 CANADA Cranberry Curd Tart Display until February 22, 2021 2020 ANNUAL 2 Chicken Schnitzel 38 A Smarter Way to Pan-Sear 74 Why and How to Grill Stone 4 Malaysian Chicken Satay Shrimp Fruit 6 All-Purpose Grilled Chicken 40 Fried Calamari 76 Consider Celery Root Breasts 42 How to Make Chana Masala 77 Roasted Carrots, No Oven 7 Poulet au Vinaigre 44 Farro and Broccoli Rabe Required 8 In Defense of Turkey Gratin 78 Braised Red Cabbage Burgers 45 Chinese Stir-Fried Tomatoes 79 Spanish Migas 10 The Best Turkey You’ll and Eggs 80 How to Make Crumpets Ever Eat 46 Everyday Lentil Dal 82 A Fresh Look at Crepes 13 Mastering Beef Wellington 48 Cast Iron Pan Pizza 84 Yeasted Doughnuts 16 The Easiest, Cleanest Way 50 The Silkiest Risotto 87 Lahmajun to Sear Steak 52 Congee 90 Getting Started with 18 Smashed Burgers 54 Coconut Rice Two Ways Sourdough Starter 20 A Case for Grilled Short Ribs 56 Occasion-Worthy Rice 92 Oatmeal Dinner Rolls 22 The Science of Stir-Frying 58 Angel Hair Done Right 94 Homemade Mayo That in a Wok 59 The Fastest Fresh Tomato Keeps 24 Sizzling Vietnamese Crepes Sauce 96 Brewing the Best Iced Tea 26 The Original Vindaloo 60 Dan Dan Mian 98 Our Favorite Holiday 28 Fixing Glazed Pork Chops 62 No-Fear Artichokes Cookies 30 Lion’s Head Meatballs 64 Hummus, Elevated 101 Pouding Chômeur 32 Moroccan Fish Tagine 66 Real Greek Salad 102 Next-Level Yellow Sheet Cake 34 Broiled Spice-Rubbed 68 Salade Lyonnaise Snapper 104 French Almond–Browned 70 Showstopper Melon Salads 35 Why You Should Butter- Butter Cakes 72 Celebrate Spring with Pea Baste Fish 106 Buttermilk Panna Cotta Salad 36 The World’s Greatest Tuna 108 The Queen of Tarts 73 Don’t Forget Broccoli Sandwich 110 DIY Recipes America’s Test Kitchen has been teaching home cooks how to be successful in the kitchen since 1993. -

Biotechnology-Inspired Solutions to Further Increase Sustainability and Healthiness in the Bakery Market
Biotechnology-Inspired Solutions to Further Increase Sustainability and Healthiness in the Bakery Market Joke A. Putseys1 DSM Food Specialties/DSM Biotechnology Center, Delft, The Netherlands products in sectors such as food, feed, and fuels. In doing so, it ABSTRACT uses renewable raw materials, making it one of the most inno- Biotechnology makes use of living organisms for the production of vative approaches to developing a circular, biobased economy. sustainable, biobased food, feed, fuel, and materials. Biocatalyst en- Many biotechnology-inspired solutions, such as enzymes, have zymes, used to improve the process or product quality of food, are a key already been developed to minimize food losses using low-car- example of how industrial biotechnology can be used to help address bon emission technologies. In addition to the functional ben- climate change and resource scarcity. In the bakery market and along efits enzymes can provide, the same technologies are applied in the baking value chain, various biotechnology-inspired solutions have the development of new products for growing markets, like or- already been implemented to reduce the carbon footprint by reduc- ganic and healthier bakery products. An overview of the general ing food waste and loss, lowering energy consumption, and creating benefits is provided in Figure 1. These benefits are listed Table I clean(er)-label products. Some challenges still remain, especially in realizing sustainable solutions for growing market trends, like healthier together with the problems they can be used to solve and poten- or organic baked products. tial feasible biotechnology-inspired solutions. Each of these top- ics will be discussed in more detail, with a focus on if and how each of these trends affects sustainability along the value chain. -

20150511 ENG Report OVAM Food Loss and Packaging
Food loss and packaging Food loss and packaging Document description 1. Title of publication Food loss and packaging 2. Responsible Publisher 6. Number of pages Danny Wille, OVAM, Stationsstraat 110, 2800 Mechelen 3. Legal deposit number 7. Number of tables and figures 4. Key words 8. Date of publication Food, packaging, materials 5. Summary 9. Price* In 2013-2014 Studio Spark, VITO en Pack4Food did research, commissioned by OVAM, on the relationship between food waste and packaging. This was done in close collaboration with a Steering Committee representing different chain actors. Finally, a communication strategy was presented. 10. Steering committee and/or author Willy Sarlee, Joke Van Cuyk, Miranda Geusens (OVAM), Gaëlle Janssens (Fost Plus), Caroline Auriel (IVCIE), Kris Roels (Departement Landbouw en Visserij), Liesje De Schamphelaire (Fevia Vlaanderen), Geraldine Verwilghen (COMEOS) 11. Contact person(s) Willy Sarlee, OVAM, Unit Policy Innovation, 015 284 298, [email protected] Gaëlle Janssens, Fost Plus, 2 775 05 68, [email protected] 12. Other publications about the same subject Voedselverlies in ketenperspectief : OVAM, 2012 Verzameling van kwantitatieve gegevens van organisch-biologisch afval horeca. Mechelen : OVAM, 2012. Nulmeting van voedselverspilling bij Vlaamse gezinnen via sorteeranalyse van het restafval. Mechelen : OVAM, 2011 Voedselverspilling: literatuurstudie. Mechelen : OVAM, 2011. Information from this document may be reproduced with due acknowledgement of the source. Most OVAM publications can be -

30 Favorite Recipes
Pamela’s 30 Favorite Recipes 30 YEARS OF CREATING DELICIOUS GLUTEN-FREE FOODS On April 1, 1988 I drove my very first forklift down the street from my family’s natural foods bakery to my own warehouse where Pamela’s Products was born. The family business was in its third generation, and up until that day, I had spent a lot of my life there, in many different jobs. I was originally a cookie packer, which was one of the few jobs available for a girl. I worked with Virgie who had six kids and whose husband Mike was our bread baker. No hairnets or gloves were worn, and there would be crumbs under my nails and I’d smell of cookies by the end of the day. With an aching back from bending over the BW6 wrapping machine six hours a day, I’d pull out the racks of heavy metal sheet pans filled with soya cookies and rice cookies. My cousin Piero, who came from Italy to work for my grandfather, was the cookie baker. Every time he pushed another rack of cookies into the packing area, I’d pray it was the last one for the day. My grandfather, father, uncle, cousins, brothers, and from time to time my sister, mom and aunt all worked there. It was truly a family business, which was one of the reasons I loved it so. In my time, I was a paper filer, a cookie packer, and a secretary. Then I started baking my own little line of bran products that I created. -
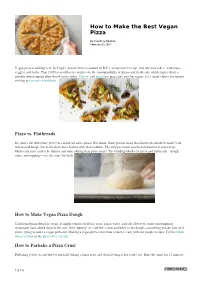
How to Make the Best Vegan Pizza
How to Make the Best Vegan Pizza By Courtney Davison February 02 2021 Vegan pizza is nothing new: In Virgil’s Aeneid (written around 30 B.C.), weary travelers top “thin wheaten cakes” with fruits, veggies, and herbs. That 2,000-year-old scene touches on the customizability of pizzas and flatbreads, which makes them a popular option among plant-based eaters today. Cheese- and meat-free pizza isn’t just for vegans; it’s a smart choice for anyone seeking to eat more healthfully. Pizza vs. Flatbreads So, what’s the difference between a flatbread and a pizza? Not much. Some purists insist that flatbreads should be made with unleavened dough, but many chefs have broken with that tradition. The only persistent practical distinction is crust-deep: Flatbread crusts tend to be thinner and more oblong than pizza crusts. The building blocks for pizza and flatbreads—dough, sauce, and toppings—are the same for both. How to Make Vegan Pizza Dough Traditional pizza dough is vegan. It simply consists of flour, yeast, sugar, water, and salt. However, some contemporary restaurants have added dairy to the mix, with “buttery” or “stuffed” crusts and whey in the dough—something to take note of if you’re trying to order a vegan pizza out. Making a vegan pizza crust from scratch is easy with our simple recipes: Try the whole wheat version or the gluten-free version. How to Parbake a Pizza Crust Parbaking refers to a method of partially baking a pizza crust and then freezing it for future use. Bake the crust for 15 minutes 1 O F 4 (continued) How to Make the Best Vegan Pizza By Courtney Davison December 19 2019 at 450°F; then freeze it. -

Molto Gusto: Easy Italian Cooking
Molto Gusto Easy Italian Cooking at Home Mario Batali and Mark Ladner Photography by Quentin Bacon Art Direction by Douglas Riccardi and Lisa Eaton THIS BOOK IS DEDICATED TO SUSI, BENNO & LEO for whom gusto is a way of life—MARIO I WOULD LIKE TO THANK MY FAMILY, CHRISTINE, RILEY & JASPER, for their support and patience—MARK Contents Introduction 1. Vegetable Antipasti 2· Seafood & Meat Antipasti 3. Bruschetta & Cheese 4· Insalata 5· Pasta 6· Pizza 7· Gelato & Sorbetto Glossary Sources Searchable Terms Acknowledgments About the Author Other Books by Mario Batali Credits Copyright About the Publisher Introduction I have written and spoken perhaps too many words about Italian food and how and why I translate its inherent excellence and deliciousness to the American table. I have been on and in several thousand television shows and explained to a very large audience the philosophy of the Italian family, the importance of the table in daily life, and the significance of regional variations and the fierce respect and love for these regional differences from town to town—and even from home to home on the same street. I have and will continue to espouse this Italian strategy, and I love to interpret it and illuminate it for the many of us who are Italians, whether or not we were born there—that is, we have ancestral roots there—and for the many of us who merely want to be Italians, at least at the dinner table. Us is a big word these days, and I do not use it lightly. Who are we? By we, I mean those like-minded individuals who seek out the delicious, the traditional, the innovative, the unique, and the geo-specific in the world of nutrition and pleasure at the table, almost always in the company of others like us or of the same mind. -

Baking Tips, Time Savers, and Kitchen Substitutions from Common Sense Home
Baking Tips, Time Savers, and Kitchen Substitutions From Common Sense Home https://commonsensehome.com Some people throw their hands up and say, "I can't bake!", but these simple baking tips, time savers, and substitutions may change your mind. We hope you find this compilation useful. Happy baking! Laurie Neverman, Common Sense Home P.S. – If homestyle recipes with troubleshooting tips are something you enjoy, check out my print book, “Never Buy Bread Again”. We cover an assortment of basic breads and quick bread type foods, including pancakes and tortillas. Plus, there are troubleshooting tips, and pointers for parbaking and freezing raw dough. Contents Pantry and Prep Baking Tips ......................................................................... 5 1. Brands Matter ........................................................................................ 5 2. Freshness Matters .................................................................................. 5 3. Read the Whole Recipe Before You Start, and Prep Ingredients ........... 6 Baking Tips for When You're Ready to Mix and Bake .................................. 6 4. Watch the Weather ................................................................................ 6 5. Get Ingredients to the Right Temperature ............................................ 7 6. Check Your Oven Temperature ............................................................. 7 7. Measure and Mix the Right Way............................................................ 7 8. Parchment Paper is your friend -
March/April 2015 Connection
The Foothills MARCH/APRIL 2015 CONNECTION COMMITTED CRUISERS Gearheads club raises money for local charities UNDER ATTACK AMISH STYLE Re-enactors besiege Local hardware store strikes deal historic Ivy Point with Amish community INDUSTRY NEWS BY SHIRLEY BLOOMFIELD, CEO LANDLINE? Rural Connections NTCA—THE RURAL BROADBAND ASSOCIatION The search for better broadband You still need should start with existing local providers one in 2015 here is no question that broad- provider, rural America now has access to band Internet service is the key to affordable broadband in some of the most Teconomic and community de- remote locations. But the sustainability of Today, mobility means everything. velopment, especially in rural America. those networks is at risk, and other areas We want to check email, log onto However, there are differing Facebook, watch videos, get the opinions in Washington about news and generally stay connected the best way to continue no matter where we are. And that, of building our nation’s con- course, includes the ability to make nected infrastructure. phone calls. With mobile phones in While I applaud President practically everyone’s pocket, some Obama’s recent attention on people question the need for a tradi- increasing every American’s Rural tional landline. But consider this: access to robust and afford- telecommunications providers are able broadband, it’s not clear With a landline, you never have delivering advanced that his focus on creating to worry about signal strength. technology to their 1 Knowing you can get a call through, more government-run net- customers. works in marketplaces where especially during an emergency, is private operators already exist more than a comfort. -
Food Processing
VATIS UPDATE Food Processing Vol. 3 No. 127 n Jan - Mar 2016 ISSN 0971-5649 Apprise yourself with the latest technological innovations Highlights l Prediction system for food safety and quality l Food additive that may prevent skin cancer l Coating extends the shelf-life of eggs l New way to make ‘green’ coffee l Eco-friendly food packaging material l Tomato-harvesting robots VATIS UPDATE: Food Processing n Jan-Mar 2016 1 The Asian and Pacific Centre for Transfer of Technology (APCTT), a subsidiary body of ESCAP, was established on 16 July 1977 with the objectives: to assist the members and associate members of ESCAP through strengthening their capabilities to develop and manage national innovation systems; develop, transfer, adapt and apply technology; improve the terms of transfer of technology; and identify and promote the development and transfer of technologies relevant to the region. The Centre will achieve the above objectives by undertaking such functions as: n Research and analysis of trends, conditions and opportunities; n Advisory services; n Dissemination of information and good practices; n Networking and partnership with international organizations and key stakeholders; and n Training of national personnel, particularly national scientists and policy analysts. The shaded areas of the map indicate ESCAP members and associate members Cover Photo Multi-commodity solar dryer (Credit: Philippine Center for Postharvest Development and Mechanization, Philippines) CONTENTS Vol. 3 No. 127 Jan-Mar 2016 VATIS* Update IN THE NEWS 4 Food Processing is published 4 times a year to keep the ¨ ASEAN countries to improve nutrition policies ¨ India issues food import norms readers up to date of most of the notice ¨ Sri Lanka increases import tax on potatoes, onions ¨ Study on palm oil relevant and latest technological in Indonesia ¨ Sri Lanka to establish first pro food test lab ¨ Food safety capacity developments and events in the field of building ¨ Viet Nam’s supplemental food market Non-conventional Energy. -

Breakfast Cookery 3N0895
Breakfast Cookery 3N0895 © 1 Learning outcomes 1. explain the basic principles of food safety and associated legislation 2. explain the organisation of a professional kitchen 3. use kitchen terminology, weights and measures appropriately 4. explain how different degrees of cooking affect foods 5. demonstrate basic techniques of tasting, recognition of food freshness and garnishing finished dishes 6. demonstrate basic cookery processes including grilling, poaching, shallow frying, boiling and simmering 7. demonstrate basic techniques for preparation of hot and cold cereals and breads 8. assist in preparing and presenting a full Irish cooked and a continental breakfast, plated and buffet style 9. comply with current food safety and hygiene legislation and regulation 10. demonstrate basic understanding of the food cost and quality control cycle 2 Food safety organisations and legislation This section covers: • The basic principles of food safety and associated legislation • How to comply with current food safety and hygiene legislation and regulation in personal and supervised work practices 3 Research Conduct your own research using leaflets from FSAI, HSA and HSE on matters relating to food safety organisations and legislation. 4 Food Safety Authority of Ireland • The FSAI was established under the Food Safety Authority of Ireland Act, 1998. This Act was enacted in July 1998 and came into effect on 1st January 1999. • The Food Safety Authority of Ireland (FSAI) functions to Examples: ensure that food purchased, distributed or marketed in Ireland meets the highest standards of food safety and • Regulation hygiene. 852/2004/EC on the • It also ensures that food complies with legal requirements, or hygiene of foodstuffs. -

Use and Care Guide
MASTERPIECE® SERIES BUILT-IN OVEN Models/Modèle/Modelo Built-in oven ME301JP Four encastré ME301JS ME302JP ME302JS MED271JS MED272JS MED301JS MED301JP MED302JS MED302JP MEDMC301JS MEDMC301JP MEDMCW71JS MEDMCW31JP Table of Contents........................... 4 MEDMCW31JS Table des Matières..............................................36 Table of ContentsUse ancare md ual About This Manual ......................................................... 5 STATEMENT OF LIMITED PRODUCT WARRANTY.... 34 How This Manual is Organized........................................... 5 What this Warranty Covers & Who it Applies to............ 34 Signal Words for Safety and Best Use.............................. 5 How Long the Warranty Lasts........................................... 34 IMPORTANT SAFETY INSTRUCTIONS ......................... 6 Repair/Replace as Your Exclusive Remedy .................. 34 Proper Installation and Maintenance ................................. 6 Out of Warranty Product .................................................... 34 Fire Safety ............................................................................... 6 Warranty Exclusions ........................................................... 34 Burn Prevention ..................................................................... 7 THERMADOR® Support .............................................. 35 Child Safety............................................................................. 7 Service.................................................................................. -

Contents in Brief Introduction to Pizza
This is the 15-page Table of Contents from The Original Encyclopizza. To return to prior page, use your Back button. ~ To get more info on this book, go to: http://correllconcepts.com/Encyclopizza/_home_encyclopizza.htm To order this book, go to: https://www.amazon.com/dp/B00XK0Q4V6 Contents in Brief Introduction to Pizza............................................................................21 Chapter 1 – On-site Preparation vs. Ready-to-Use...........................25 Chapter 2 – Mixers and Food Processing Equipment......................39 Chapter 3 – Dough Ingredients.........................................................105 Chapter 4 – Dough-making ...............................................................165 Chapter 5 – Dough Management......................................................225 Chapter 6 – Dough and Crust Troubleshooting..............................233 Chapter 7 – Sauce..............................................................................265 Chapter 8 – Cheese............................................................................319 Chapter 9 – Toppings ........................................................................359 Chapter 10 – Commissary vs. On-site Preparation ........................409 Chapter 11 – Ordering, Receiving, and Storage .............................417 Chapter 12 – Measurements and Conversions...............................437 ~ See next page for Contents in Detail ~ 5 Contents in Detail Introduction to Pizza...........................................................................