Mycologia Obscura: Hidden and Layered Realms of Fungal Diversity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

First Record of the Genus Ilyomyces for North America, Parasitizing Stenus Clavicornis
Bulletin of Insectology 66 (2): 269-272, 2013 ISSN 1721-8861 First record of the genus Ilyomyces for North America, parasitizing Stenus clavicornis Danny HAELEWATERS Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, USA Abstract The ectoparasitic fungus Ilyomyces cf. mairei (Ascomycota Laboulbeniales) is reported for the first time outside Europe on the rove beetle Stenus clavicornis (Coleoptera Staphylinidae). This record is the first for the genus Ilyomyces in North America. De- scription, illustrations, and discussion in relation to the different species in the genus are given. Key words: ectoparasites, François Picard, Ilyomyces, rove beetles, Stenus. Introduction 1939) described Acallomyces lavagnei F. Picard (Picard, 1913), which he later reassigned to a new genus Ilyomy- Fungal diversity is under-documented, with diversity ces while adding a second species, Ilyomyces mairei F. estimates often based only on relationships with plants. Picard (Picard, 1917). For a long time both species were Meanwhile, the estimated number of fungi associated only known from France, until Santamaría (1992) re- with insects ranges from 10,000 to 50,000, most of ported I. mairei from Spain. Weir (1995) added two which still need be described from the unexplored moist more species to the genus: Ilyomyces dianoi A. Weir and tropical regions (Weir and Hammond, 1997). Despite Ilyomyces victoriae A. Weir, parasitic on Steninae from the biological and ecological importance the relation- Sulawesi, Indonesia. This paper presents the first record ship might have for studies of co-evolution of host and of Ilyomyces for the New World. parasite and in applications in biological control, insect- parasites have received little attention, unfortunately. -

Synopsis of the Tribe Platynini in New Zealand (Coleoptera: Carabidae)
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Center for Systematic Entomology, Gainesville, Insecta Mundi Florida 3-30-2021 Synopsis of the tribe Platynini in New Zealand (Coleoptera: Carabidae) André Larochelle Marie-Claude Larivière Follow this and additional works at: https://digitalcommons.unl.edu/insectamundi Part of the Ecology and Evolutionary Biology Commons, and the Entomology Commons This Article is brought to you for free and open access by the Center for Systematic Entomology, Gainesville, Florida at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Insecta Mundi by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. A journal of world insect systematics INSECTA MUNDI 0864 Synopsis of the tribe Platynini in New Zealand Page Count: 96 (Coleoptera: Carabidae) André Larochelle and Marie-Claude Larivière Larochelle and Larivière New Zealand Arthropod Collection, Manaaki Whenua–Landcare Research, Private Bag 92170, Auckland 1142, New Zealand Prosphodrus waimana Larochelle and Larivière, new species Date of issue: April 30, 2021 Center for Systematic Entomology, Inc., Gainesville, FL Larochelle A, Larivière M-C. 2021. Synopsis of the tribe Platynini in New Zealand (Coleoptera: Carabidae). Insecta Mundi 0864: 1–96. Published on April 30, 2021 by Center for Systematic Entomology, Inc. P.O. Box 141874 Gainesville, FL 32614-1874 USA http://centerforsystematicentomology.org/ Insecta Mundi is a journal primarily devoted to insect systematics, but articles can be published on any non- marine arthropod. Topics considered for publication include systematics, taxonomy, nomenclature, checklists, faunal works, and natural history. Insecta Mundi will not consider works in the applied sciences (i.e. medi- cal entomology, pest control research, etc.), and no longer publishes book reviews or editorials. -

A Catalogue of Coleoptera Specimens with Potential Forensic Interest in the Goulandris Natural History Museum Collection
ENTOMOLOGIA HELLENICA Vol. 25, 2016 A catalogue of Coleoptera specimens with potential forensic interest in the Goulandris Natural History Museum collection Dimaki Maria Goulandris Natural History Museum, 100 Othonos St. 14562 Kifissia, Greece Anagnou-Veroniki Maria Makariou 13, 15343 Aghia Paraskevi (Athens), Greece Tylianakis Jason Zoology Department, University of Canterbury, Private Bag 4800, Christchurch, New Zealand http://dx.doi.org/10.12681/eh.11549 Copyright © 2017 Maria Dimaki, Maria Anagnou- Veroniki, Jason Tylianakis To cite this article: Dimaki, M., Anagnou-Veroniki, M., & Tylianakis, J. (2016). A catalogue of Coleoptera specimens with potential forensic interest in the Goulandris Natural History Museum collection. ENTOMOLOGIA HELLENICA, 25(2), 31-38. doi:http://dx.doi.org/10.12681/eh.11549 http://epublishing.ekt.gr | e-Publisher: EKT | Downloaded at 27/12/2018 06:22:38 | ENTOMOLOGIA HELLENICA 25 (2016): 31-38 Received 15 March 2016 Accepted 12 December 2016 Available online 3 February 2017 A catalogue of Coleoptera specimens with potential forensic interest in the Goulandris Natural History Museum collection MARIA DIMAKI1’*, MARIA ANAGNOU-VERONIKI2 AND JASON TYLIANAKIS3 1Goulandris Natural History Museum, 100 Othonos St. 14562 Kifissia, Greece 2Makariou 13, 15343 Aghia Paraskevi (Athens), Greece 3Zoology Department, University of Canterbury, Private Bag 4800, Christchurch, New Zealand ABSTRACT This paper presents a catalogue of the Coleoptera specimens in the Goulandris Natural History Museum collection that have potential forensic interest. Forensic entomology can help to estimate the time elapsed since death by studying the necrophagous insects collected on a cadaver and its surroundings. In this paper forty eight species (369 specimens) are listed that belong to seven families: Silphidae (3 species), Staphylinidae (6 species), Histeridae (11 species), Anobiidae (4 species), Cleridae (6 species), Dermestidae (14 species), and Nitidulidae (4 species). -

Studies of the Laboulbeniomycetes: Diversity, Evolution, and Patterns of Speciation
Studies of the Laboulbeniomycetes: Diversity, Evolution, and Patterns of Speciation The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:40049989 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA ! STUDIES OF THE LABOULBENIOMYCETES: DIVERSITY, EVOLUTION, AND PATTERNS OF SPECIATION A dissertation presented by DANNY HAELEWATERS to THE DEPARTMENT OF ORGANISMIC AND EVOLUTIONARY BIOLOGY in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the subject of Biology HARVARD UNIVERSITY Cambridge, Massachusetts April 2018 ! ! © 2018 – Danny Haelewaters All rights reserved. ! ! Dissertation Advisor: Professor Donald H. Pfister Danny Haelewaters STUDIES OF THE LABOULBENIOMYCETES: DIVERSITY, EVOLUTION, AND PATTERNS OF SPECIATION ABSTRACT CHAPTER 1: Laboulbeniales is one of the most morphologically and ecologically distinct orders of Ascomycota. These microscopic fungi are characterized by an ectoparasitic lifestyle on arthropods, determinate growth, lack of asexual state, high species richness and intractability to culture. DNA extraction and PCR amplification have proven difficult for multiple reasons. DNA isolation techniques and commercially available kits are tested enabling efficient and rapid genetic analysis of Laboulbeniales fungi. Success rates for the different techniques on different taxa are presented and discussed in the light of difficulties with micromanipulation, preservation techniques and negative results. CHAPTER 2: The class Laboulbeniomycetes comprises biotrophic parasites associated with arthropods and fungi. -

De La Península Ibérica Catalogue of the Carabidae (Coleoptera) of the Iberian Peninsula
Catálogo de los Carabidae (Coleoptera) de la Península Ibérica Catalogue of the Carabidae (Coleoptera) of the Iberian Peninsula José Serrano MONOGRAFÍAS SEA, vol. 9 ZARAGOZA, 2003 a Dedicatoria A mi mujer, Bárbara y a mis hijos José Enrique y Antonio. Por todo. Índice Prólogo ......................................................................... 5 Agradecimiento................................................................... 6 Notas a las distintas partes de la obra................................................... 7 El catálogo...................................................................... 11 Cambio nomenclatural ............................................................ 82 Las novedades................................................................... 83 Relación sintética de la Sistemática empleada y estadísticas del catálogo....................... 85 Los mapas de las regiones naturales de la Península Ibérica................................. 91 La bibliografía ................................................................... 93 Índice taxonómico............................................................... 107 Index Foreword........................................................................ 5 Acknowledgements................................................................ 6 Notes to the the chapters of this work .................................................. 7 The catalogue ................................................................... 11 Proposal of nomenclatural change................................................... -

The Beetle Fauna of Dominica, Lesser Antilles (Insecta: Coleoptera): Diversity and Distribution
INSECTA MUNDI, Vol. 20, No. 3-4, September-December, 2006 165 The beetle fauna of Dominica, Lesser Antilles (Insecta: Coleoptera): Diversity and distribution Stewart B. Peck Department of Biology, Carleton University, 1125 Colonel By Drive, Ottawa, Ontario K1S 5B6, Canada stewart_peck@carleton. ca Abstract. The beetle fauna of the island of Dominica is summarized. It is presently known to contain 269 genera, and 361 species (in 42 families), of which 347 are named at a species level. Of these, 62 species are endemic to the island. The other naturally occurring species number 262, and another 23 species are of such wide distribution that they have probably been accidentally introduced and distributed, at least in part, by human activities. Undoubtedly, the actual numbers of species on Dominica are many times higher than now reported. This highlights the poor level of knowledge of the beetles of Dominica and the Lesser Antilles in general. Of the species known to occur elsewhere, the largest numbers are shared with neighboring Guadeloupe (201), and then with South America (126), Puerto Rico (113), Cuba (107), and Mexico-Central America (108). The Antillean island chain probably represents the main avenue of natural overwater dispersal via intermediate stepping-stone islands. The distributional patterns of the species shared with Dominica and elsewhere in the Caribbean suggest stages in a dynamic taxon cycle of species origin, range expansion, distribution contraction, and re-speciation. Introduction windward (eastern) side (with an average of 250 mm of rain annually). Rainfall is heavy and varies season- The islands of the West Indies are increasingly ally, with the dry season from mid-January to mid- recognized as a hotspot for species biodiversity June and the rainy season from mid-June to mid- (Myers et al. -

Influence of Plant Parameters on Occurrence and Abundance Of
HORTICULTURAL ENTOMOLOGY Influence of Plant Parameters on Occurrence and Abundance of Arthropods in Residential Turfgrass 1 S. V. JOSEPH AND S. K. BRAMAN Department of Entomology, College of Agricultural and Environmental Sciences, University of Georgia, 1109 Experiment Street, GrifÞn, GA 30223-1797 J. Econ. Entomol. 102(3): 1116Ð1122 (2009) ABSTRACT The effect of taxa [common Bermuda grass, Cynodon dactylon (L.); centipedegrass, Eremochloa ophiuroides Munro Hack; St. Augustinegrass, Stenotaphrum secundatum [Walt.] Kuntze; and zoysiagrass, Zoysia spp.], density, height, and weed density on abundance of natural enemies, and their potential prey were evaluated in residential turf. Total predatory Heteroptera were most abundant in St. Augustinegrass and zoysiagrass and included Anthocoridae, Lasiochilidae, Geocoridae, and Miridae. Anthocoridae and Lasiochilidae were most common in St. Augustinegrass, and their abundance correlated positively with species of Blissidae and Delphacidae. Chinch bugs were present in all turf taxa, but were 23Ð47 times more abundant in St. Augustinegrass. Anthocorids/lasiochilids were more numerous on taller grasses, as were Blissidae, Delphacidae, Cicadellidae, and Cercopidae. Geocoridae and Miridae were most common in zoysiagrass and were collected in higher numbers with increasing weed density. However, no predatory Heteroptera were affected by grass density. Other beneÞcial insects such as staphylinids and parasitic Hymenoptera were captured most often in St. Augustinegrass and zoysiagrass. These differences in abundance could be in response to primary or alternate prey, or reßect the inßuence of turf microenvironmental characteristics. In this study, SimpsonÕs diversity index for predatory Heteroptera showed the greatest diversity and evenness in centipedegrass, whereas the herbivores and detritivores were most diverse in St. Augustinegrass lawns. These results demonstrate the complex role of plant taxa in structuring arthropod communities in turf. -

Insects and Molluscs, According to the Procedures Outlined Below
Bush Blitz – ACT Expedition 26 Nov – 6 Dec 2018 ACT Expedition Bush Blitz Hemiptera, Hymenoptera, Lepidoptera, Orthoptera, Terrestrial molluscs 26 Nov – 6 Dec 2018 Submitted: 5 April 2019 Debbie Jennings and Olivia Evangelista Nomenclature and taxonomy used in this report is consistent with: The Australian Faunal Directory (AFD) http://www.environment.gov.au/biodiversity/abrs/online-resources/fauna/afd/home Page 1 of 43 Bush Blitz – ACT Expedition 26 Nov – 6 Dec 2018 Contents Contents .................................................................................................................................. 2 List of contributors ................................................................................................................... 3 Abstract ................................................................................................................................... 4 1. Introduction ...................................................................................................................... 4 2. Methods .......................................................................................................................... 6 2.1 Site selection ............................................................................................................. 6 2.2 Survey techniques ..................................................................................................... 6 2.2.1 Methods used at standard survey sites ................................................................... 7 2.3 Identifying -

New and Interesting <I>Laboulbeniales</I> From
ISSN (print) 0093-4666 © 2014. Mycotaxon, Ltd. ISSN (online) 2154-8889 MYCOTAXON http://dx.doi.org/10.5248/129.439 Volume 129(2), pp. 439–454 October–December 2014 New and interesting Laboulbeniales from southern and southeastern Asia D. Haelewaters1* & S. Yaakop2 1Farlow Reference Library and Herbarium of Cryptogamic Botany, Harvard University 22 Divinity Avenue, Cambridge, Massachusetts 02138, U.S.A. 2Faculty of Science & Technology, School of Environmental and Natural Resource Sciences, Universiti Kebangsaan Malaysia, Bangi 43600, Malaysia * Correspondence to: [email protected] Abstract — Two new species of Laboulbenia from the Philippines are described and illustrated: Laboulbenia erotylidarum on an erotylid beetle (Coleoptera, Erotylidae) and Laboulbenia poplitea on Craspedophorus sp. (Coleoptera, Carabidae). In addition, we present ten new records of Laboulbeniales from several countries in southern and southeastern Asia on coleopteran hosts. These are Blasticomyces lispini from Borneo (Indonesia), Cantharomyces orientalis from the Philippines, Dimeromyces rugosus on Leiochrodes sp. from Sumatra (Indonesia), Laboulbenia anoplogenii on Clivina sp. from India, L. cafii on Remus corallicola from Singapore, L. satanas from the Philippines, L. timurensis on Clivina inopaca from Papua New Guinea, Monoicomyces stenusae on Silusa sp. from the Philippines, Ormomyces clivinae on Clivina sp. from India, and Peyritschiella princeps on Philonthus tardus from Lombok (Indonesia). Key words — Ascomycota, insect-associated fungi, morphology, museum collection study, Roland Thaxter, taxonomy Introduction One group of microscopic insect-associated parasitic fungi, the order Laboulbeniales (Ascomycota, Pezizomycotina, Laboulbeniomycetes), is perhaps the most intriguing and yet least studied of all entomogenous fungi. Laboulbeniales are obligate parasites on invertebrate hosts, which include insects (mainly beetles and flies), millipedes, and mites. -

The Fungi Constitute a Major Eukary- Members of the Monophyletic Kingdom Fungi ( Fig
American Journal of Botany 98(3): 426–438. 2011. T HE FUNGI: 1, 2, 3 … 5.1 MILLION SPECIES? 1 Meredith Blackwell 2 Department of Biological Sciences; Louisiana State University; Baton Rouge, Louisiana 70803 USA • Premise of the study: Fungi are major decomposers in certain ecosystems and essential associates of many organisms. They provide enzymes and drugs and serve as experimental organisms. In 1991, a landmark paper estimated that there are 1.5 million fungi on the Earth. Because only 70 000 fungi had been described at that time, the estimate has been the impetus to search for previously unknown fungi. Fungal habitats include soil, water, and organisms that may harbor large numbers of understudied fungi, estimated to outnumber plants by at least 6 to 1. More recent estimates based on high-throughput sequencing methods suggest that as many as 5.1 million fungal species exist. • Methods: Technological advances make it possible to apply molecular methods to develop a stable classifi cation and to dis- cover and identify fungal taxa. • Key results: Molecular methods have dramatically increased our knowledge of Fungi in less than 20 years, revealing a mono- phyletic kingdom and increased diversity among early-diverging lineages. Mycologists are making signifi cant advances in species discovery, but many fungi remain to be discovered. • Conclusions: Fungi are essential to the survival of many groups of organisms with which they form associations. They also attract attention as predators of invertebrate animals, pathogens of potatoes and rice and humans and bats, killers of frogs and crayfi sh, producers of secondary metabolites to lower cholesterol, and subjects of prize-winning research. -
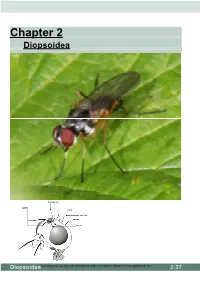
Chapter 2 Diopsoidea
Chapter 2 Diopsoidea DiopsoideaTeaching material only, not intended for wider circulation. [email protected] 2:37 Diptera: Acalyptrates DIOPSOI D EA 50: Tanypezidae 53 ------ Base of tarsomere 1 of hind tarsus very slightly projecting ventrally; male with small stout black setae on hind trochanter and posterior base of hind femur. Postocellar bristles strong, at least half as long as upper orbital seta; one dorsocentral and three orbital setae present Tanypeza ----------------------------------------- 55 2 spp.; Maine to Alberta and Georgia; Steyskal 1965 ---------- Base of tarsomere 1 of hind tarsus strongly projecting ventrally, about twice as deep as remainder of tarsomere 1 (Fig. 3); male without special setae on hind trochanter and hind femur. Postocellar bristles weak, less than half as long as upper orbital bristle; one to three dor socentral and zero to two orbital bristles present non-British ------------------------------------------ 54 54 ------ Only one orbital bristle present, situated at top of head; one dorsocentral bristle present --------------------- Scipopeza Enderlein Neotropical ---------- Two or three each of orbital and dorsocentral bristles present ---------------------Neotanypeza Hendel Neotropical Tanypeza Fallén, 1820 One species 55 ------ A black species with a silvery patch on the vertex and each side of front of frons. Tho- rax with notopleural depression silvery and pleurae with silvery patches. Palpi black, prominent and flat. Ocellar bristles small; two pairs of fronto orbital bristles; only one (outer) pair of vertical bristles. Frons slightly narrower in the male than in the female, but not with eyes almost touching). Four scutellar, no sternopleural, two postalar and one supra-alar bristles; (the anterior supra-alar bristle not present). Wings with upcurved discal cell (11) as in members of the Micropezidae. -

Insects in Kansas Book: 2016 Revised Taxonomy
Insects in Kansas Book: 2016 Revised Taxonomy TAXONOMIC CHANGES TO INSECT ORDERS 1. Order Collembola, Springtails, are no longer classified as insects (pg. 38‐39) but were elevated to Class status 2. Order Thysanura, Bristletails and Silverfish are now split into their own Orders and Thysanura no longer exists as an Order name. a. Bristletails=Order Microcoryphia (pg. 40‐41) b. Silverfish=Order Zygentoma (pg. 40‐41) 3. Order Phasmatodea, walkingsticks, are sometimes referred to as Phasmida (pg. 55) 4. Order Blattodea now includes both cockroaches and termites (Order Isoptera is now a suborder) (pg. 84‐85) 5. Order Pscoptera, barklice, are now called Psocodea(pg. 86) 6. Orders Mallophaga and Anoplura (pg. 87‐92) are now in Psocodea as suborders 7. Order Hemiptera now includes the Order Homoptera (Homoptera is now a suborder) (pg. 121‐ 418) 8. Order Neuroptera now includes lacewings, owlflies, and antlions but NOT dobsonflies (see #9) (pg. 153‐159) 9. Order Megaloptera now includes dobsonflies, alderflies, and fishflies (pg. 153‐159) TAXONOMIC CHANGES TO INSECT FAMILIES 1. Cave and camel crickets are now in the Family Rhapidophoridae not Gryllacrididae (pg. 73) 2. Ant crickets have been put into their own Family now called Myrmecophilidae (pg. 74‐75) 3. Red‐legged Earwigs are now in the Family Anisolabididae (pg. 78) 4. German and wood cockroaches are now in the Family Ectobiidae, F. Blattellidae is no longer valid (pg. 82‐83) 5. Family Liposcelidae is now called Liposcelididae (pg. 86) 6. Pubic lice are now in their own Family Phthiridae (pg. 92) 7. Family Pentatomidae no longer includes the Shield bugs or shield‐backed bugs (pg.