Diseases of Bulbous Iris
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE
Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE LILIACEAE de Jussieu 1789 (Lily Family) (also see AGAVACEAE, ALLIACEAE, ALSTROEMERIACEAE, AMARYLLIDACEAE, ASPARAGACEAE, COLCHICACEAE, HEMEROCALLIDACEAE, HOSTACEAE, HYACINTHACEAE, HYPOXIDACEAE, MELANTHIACEAE, NARTHECIACEAE, RUSCACEAE, SMILACACEAE, THEMIDACEAE, TOFIELDIACEAE) As here interpreted narrowly, the Liliaceae constitutes about 11 genera and 550 species, of the Northern Hemisphere. There has been much recent investigation and re-interpretation of evidence regarding the upper-level taxonomy of the Liliales, with strong suggestions that the broad Liliaceae recognized by Cronquist (1981) is artificial and polyphyletic. Cronquist (1993) himself concurs, at least to a degree: "we still await a comprehensive reorganization of the lilies into several families more comparable to other recognized families of angiosperms." Dahlgren & Clifford (1982) and Dahlgren, Clifford, & Yeo (1985) synthesized an early phase in the modern revolution of monocot taxonomy. Since then, additional research, especially molecular (Duvall et al. 1993, Chase et al. 1993, Bogler & Simpson 1995, and many others), has strongly validated the general lines (and many details) of Dahlgren's arrangement. The most recent synthesis (Kubitzki 1998a) is followed as the basis for familial and generic taxonomy of the lilies and their relatives (see summary below). References: Angiosperm Phylogeny Group (1998, 2003); Tamura in Kubitzki (1998a). Our “liliaceous” genera (members of orders placed in the Lilianae) are therefore divided as shown below, largely following Kubitzki (1998a) and some more recent molecular analyses. ALISMATALES TOFIELDIACEAE: Pleea, Tofieldia. LILIALES ALSTROEMERIACEAE: Alstroemeria COLCHICACEAE: Colchicum, Uvularia. LILIACEAE: Clintonia, Erythronium, Lilium, Medeola, Prosartes, Streptopus, Tricyrtis, Tulipa. MELANTHIACEAE: Amianthium, Anticlea, Chamaelirium, Helonias, Melanthium, Schoenocaulon, Stenanthium, Veratrum, Toxicoscordion, Trillium, Xerophyllum, Zigadenus. -

BURIED TREASURE Summer 2019 Rannveig Wallis, Llwyn Ifan, Porthyrhyd, Carmarthen, UK
BURIED TREASURE Summer 2019 Rannveig Wallis, Llwyn Ifan, Porthyrhyd, Carmarthen, UK. SA32 8BP Email: [email protected] I am still trying unsuccessfully to retire from this enterprise. In order to reduce work, I am sowing fewer seeds and concentrating on selling excess stock which has been repotted in the current year. Some are therefore in quite small numbers. I hope that you find something of interest and order early to avoid any disappointments. Please note that my autumn seed list is included below. This means that seed is fresher and you can sow it earlier. Terms of Business: I can accept payment by either: • Cheque made out to "R Wallis" (n.b. Please do not fill in the amount but add the words “not to exceed £xx” ACROSS THE TOP); • PayPal, please include your email address with the order and wait for an invoice after I dispatch your order; • In cash (Sterling, Euro or US dollar are accepted, in this case I advise using registered mail). Please note that I can only accept orders placed before the end of August. Parcels will be dispatched at the beginning of September. If you are going to be away please let me know so that I can coordinate dispatch. I will not cash your cheque until your order is dispatched. If ordering by email, and following up by post, please ensure that you tick the box on the order form to avoid duplication. Acis autumnalis var pulchella A Moroccan version of this excellent early autumn flowerer. It is quite distinct in the fact that the pedicels and bracts are green rather than maroon as in the type variety. -

Narcissus Juncifolius
Report under the Article 17 of the Habitats Directive European Environment Period 2007-2012 Agency European Topic Centre on Biological Diversity Narcissus juncifolius Annex V Priority No Species group Vascular plants Regions Alpine, Atlantic, Mediterranean Narcissus juncifolius is endemic to France, occuring in Alpine, Atlantic and Mediterranean regions. The taxonomy of this taxon is not clear. According to the information on the IUCN red list website the synonym species Narcissus assoanus ssp. praelongus is present in Spain. However, Spain did not report the species. In the IUCN red list the species is assessed as Least Concern (LC) with stable population trend. It is not assessed in the French red list (2012). The overall conclusion in the Alpine region is "Unfavorable Inadequate" due to a negative population trend. It occurs with a population of 1000-5000 individuals. The main distribution area of Narcissus juncifolius is in the Mediterranean region of France. However, the population is "Unknown", but assessed as "Favorable". As in the previous report, the conservation status is "Favorable" in all components. In the French Atlantic region is assessed as "Favorable" in all components (reported for the first time). The species occurs with a population of 10000-50000 individuals Main threats (mainly low rank) are grazing, cultivation, urbanisation, modification of cultural practices and mining. No changes in overall conservation status between 2001-06 and 2007-12 reports in Alpine and Mediterranean region. The species was not reported -

Developmental Regulation of the Expression of Amaryllidaceae Alkaloid Biosynthetic Genes in Narcissus Papyraceus
G C A T T A C G G C A T genes Article Developmental Regulation of the Expression of Amaryllidaceae Alkaloid Biosynthetic Genes in Narcissus papyraceus Tarun Hotchandani 1, Justine de Villers 1 and Isabel Desgagné-Penix 1,2,* 1 Department of Chemistry, Biochemistry and Physics, Université du Québec à Trois-Rivières, 3351 boulevard des Forges, Trois-Rivières, QC G9A 5H7, Canada 2 Plant Biology Research Group, Trois-Rivières, QC G9A 5H7, Canada * Correspondence: [email protected]; Tel.: +1-819-376-5011 Received: 6 July 2019; Accepted: 5 August 2019; Published: 7 August 2019 Abstract: Amaryllidaceae alkaloids (AAs) have multiple biological effects, which are of interest to the pharmaceutical industry. To unleash the potential of Amaryllidaceae plants as pharmaceutical crops and as sources of AAs, a thorough understanding of the AA biosynthetic pathway is needed. However, only few enzymes in the pathway are known. Here, we report the transcriptome of AA-producing paperwhites (Narcissus papyraceus Ker Gawl). We present a list of 21 genes putatively encoding enzymes involved in AA biosynthesis. Next, a cDNA library was created from 24 different samples of different parts at various developmental stages of N. papyraceus. The expression of AA biosynthetic genes was analyzed in each sample using RT-qPCR. In addition, the alkaloid content of each sample was analyzed by HPLC. Leaves and flowers were found to have the highest abundance of heterocyclic compounds, whereas the bulb, the lowest. Lycorine was also the predominant AA. The gene expression results were compared with the heterocyclic compound profiles for each sample. In some samples, a positive correlation was observed between the gene expression levels and the amount of compounds accumulated. -

Forcing Guide / Forcing Guide / Narcissus Narcissus Narcissus
Forcing Guide / Narcissus 1. Methods of cultivation ..........................................................................................................2 2. Greenhouse, forcing trays and rooting media.........................................................................4 3. Choice and receipt of bulbs ..................................................................................................5 4. Forcing 9°c (pre-cooled) and un-cooled narcissi in trays........................................................ 6 5. Forcing 9°c (pre-cooled) and un-cooled narcissi in the border soil of the greenhouse.............. 9 6. Procedures for forcing in trays and cultivation in the border soil of the greenhouse ............... 11 7. Other narcissus.................................................................................................................. 14 8. Crop protection, diseases and disorders ............................................................................. 16 1 1. Methods of cultivation Introduction: The name The name ‘narcissus’ is derived from the Greek word ‘narkaein’, meaning paralysed or numbed. Narcissus was a beautiful, proud young man in Greek mythology. Too proud to return the love of women, the envy of those scorned led to his downfall. While out hunting one day, he stopped to refresh himself at a spring. Seeing his image reflected in the water, he fell in love with it and pined away for his unattainable love, until there was nothing left but a beautiful narcissus. The narcissi like the Hippeastrum -

Paeonias, Tulips, Hyacinths, Narcissus, Iris for Fall Planting
Historic, Archive Document Do not assume content reflects current scientific knowledge, policies, or practices. I * JUL 15 1$0 * Glenwood Nursery** Rochester, N. Y. V cAutumn 1913 ;Qaeonias T3ulips hyacinths Qarcissus For Fall Iris, Etc. Planting Paeonias Our selection of Paeonias will give you continuous bloom for three months. Our Collection includes the very choicest sorts—the most striking colors—white, pink and crimson in various shades. All varieties strictly true to description, all in strong divisions, with from three to five eyes. 35c each; $3.50 per dozen; $25.00 per hundred (except where otherwise noted) Alba Lutea— Pure white, with yellowish center, perfectly Mr. Chas. Levique— Exquisite salmon pink. 75c each; $6.00 globular, and sweet. per doz. Admiral de Ruyter—Splendid, brilliant red. Mad. Barillet Deschampes—Very tender pink, bordered Albatre—Ivory white. white; vigorous. Alex. Dumas— Large, bright rose flowers; very attractive. Ornement des Massifs— Flesh color; very late. Belle Francoise—Clear bright pink. Paul Kruger—Cream white, short, desirable for edging. Berlioz— Big full flowers, bright currant red. Pink Beauty—Lilac rose; decidedly a grand variety. Carmen Sylvia— Pink, dark shaded; fine. Pres. Carnot—Dark pink, light bordered. Comte Legrelle— Purple red, very large; one of the best. Reine de France—Light rose, yellow center Carmea Elegans— Flowers large, dark pink, yellowish cen- petals fringed. ters, Paeonia Officinalis Duchess de Nemours—White, yellow shaded. Distinction Large, cup shaped, violet red. — These are the old-fashioned Paeony so much loved by Festiva, Maxima— Pure white, center petals sometimes tinged rival the rose in color in with red; most desirable for cutting. -

Nomenclatural Notes on Some Autumn Flowering Daffodils (Narcissus, Amaryllidaceae)
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/314088340 Nomenclatural notes on some autumn flowering daffodils (Narcissus, Amaryllidaceae) Article in Phytotaxa · February 2017 DOI: 10.11646/phytotaxa.297.2.3 CITATIONS READS 0 159 3 authors, including: Maarten Christenhusz Plant Gateway 130 PUBLICATIONS 1,547 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Ferns and lycophytes from Paraná View project Teaching Plant Systematics View project All content following this page was uploaded by Maarten Christenhusz on 24 March 2017. The user has requested enhancement of the downloaded file. Phytotaxa 297 (2): 157–167 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2017 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.297.2.3 Nomenclatural notes on some autumn flowering daffodils (Narcissus, Amaryllidaceae) HAROLD KOOPOWITZ1*, MARILYNN HOWE2 & MAARTEN J. M. CHRISTENHUSZ3, 4 1Ecology and Evolutionary Biology, University of California, Irvine, California 92705, U.S.A; e-mail: [email protected] 2P.O. Box 11372, Marina del Rey, California 90295, U.S.A. 3Plant Gateway, Hertford, Hertfordshire, U.K. 4Royal Botanic Gardens, Kew, Richmond, Surrey, U.K. *author for correspondence Keyword: Taxonomic key, historical confusion, Narcissus obsoletus, Narcissus miniatus, Narcissus serotinus Autumn flowering daffodils have been recorded in taxonomic history for at least 500 years. First descriptions were often merely based on hearsay, as they were often not studied alive. Consequently, many errors in interpretation of these names have occurred and taxonomic confusion, errors in identification and misapplication of names have led to an accumulation of taxonomic entanglement in Narcissus Linnaeus (1753: 289) nomenclature. -
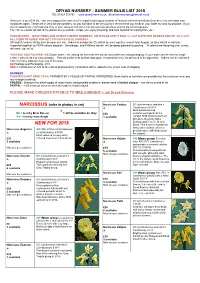
Dryad Nursery, SUMMER 2018 Bulb List
DRYAD NURSERY - SUMMER BULB LIST 2018 TEL 01423 358791 www.dryad-home.co.uk Email [email protected] Welcome to my 2018 list. I am very happy to be able to offer a particularly good number of famous Irish breeder Brian Duncan's very desirable new miniatures again. These are in very limited numbers, so you will have to be very quick! I recommend you email in your order as soon as possible. If you wish to telephone, I will note the time of your request and slot it into the appropriate place among the email requests. You can see better photos of the plants on my website. I hope you enjoy browsing, and look forward to hearing from you. PLEASE NOTE – MOST ITEMS ARE IN VERY LIMITED NUMBERS, SO PLEASE APPLY EARLY, LAST DATE FOR ORDERS END OF JULY 2017 ALL ITEMS OFFERED SUBJECT TO SUCCESSFUL HARVEST. UK and EU orders will be sent as soon as I can. Orders to outside the EU will all be sent together end of July/ August, as they will all need to be inspected together by FERA before dispatch. Snowdrops, and Fritillaria davidii will be damp-packed for posting. All plants are flowering size, unless otherwise specified. POSTAGE AND PACKING- -£5-50 per order. I am asking for less than the actual cost of delivery and packaging. If your order can be sent as Large Letter, I will refund any extra postage. Parcels will be sent by first class post, recorded delivery, so will need to be signed for. Orders can be collected from my home address if you are in the area. -

Plant Catalog
Fall PLANT & BULB SALE PLANT CATALOG FRIDAY SEPTEMBER 28 SATURDAY SEPTEMBER 29 9 A.M. – 5 P.M. Members receive a FREE ADMISSION 10% DISCOUNT on all purchases. PRESENTING SPONSOR 10th & York Street ASSOCIATE SPONSORS botanicgardens.org TABLE OF CONTENTS ADMISSION & MEMBERSHIP Bulbs 1 Entry to Fall Plant & Bulb Sale is free on Saturday and Sunday. Visit botanicgardens.org Houseplants 9 for more information. Grown at the Gardens 10 Gardens members receive 10% off their purchases but must show their membership Pansies 11 card to receive the discount. Not a member yet? Stop by the Visitor Center before Notes 12 you shop and see our Visitor Services team! REFUND POLICY BRING YOUR WAGON! All products purchased at Fall Plant & Bulb We highly encourage guests to bring their own wagons, Sale are non-refundable. wheelbarrows or carts to allow for ease of transportation. We will have limited carts and baskets for use at the sale. FOOD & BEVERAGE THE SHOP AT THE GARDENS Offshoots Café and The Hive Garden Bistro will • Your garden needs more than just plants! Stop by the Shop at the Gardens for gardening equipment, outdoor décor, fountains, pots and more. be open featuring full menus throughout • Open during Fall Plant & Bulb Sale. the sale. • Members receive 10% off all Shop purchases. PARKING, TRANSPORTATION, AND PLANT VALET • After exiting the Gardens, leave your cart at Plant Valet in front of the Visitor CONCERNED ABOUT Center while you retrieve your vehicle. Vehicle loading is permitted in the NEONICOTINOIDS? parking cutout on York Street just south of the Visitor Center. The plants grown by Denver Botanic Gardens • Free parking is located across from the Visitor Center in the Gardens’ parking for sale at Fall Plant & Bulb Sale have not been garage. -

Field Identification of the 50 Most Common Plant Families in Temperate Regions
Field identification of the 50 most common plant families in temperate regions (including agricultural, horticultural, and wild species) by Lena Struwe [email protected] © 2016, All rights reserved. Note: Listed characteristics are the most common characteristics; there might be exceptions in rare or tropical species. This compendium is available for free download without cost for non- commercial uses at http://www.rci.rutgers.edu/~struwe/. The author welcomes updates and corrections. 1 Overall phylogeny – living land plants Bryophytes Mosses, liverworts, hornworts Lycophytes Clubmosses, etc. Ferns and Fern Allies Ferns, horsetails, moonworts, etc. Gymnosperms Conifers, pines, cycads and cedars, etc. Magnoliids Monocots Fabids Ranunculales Rosids Malvids Caryophyllales Ericales Lamiids The treatment for flowering plants follows the APG IV (2016) Campanulids classification. Not all branches are shown. © Lena Struwe 2016, All rights reserved. 2 Included families (alphabetical list): Amaranthaceae Geraniaceae Amaryllidaceae Iridaceae Anacardiaceae Juglandaceae Apiaceae Juncaceae Apocynaceae Lamiaceae Araceae Lauraceae Araliaceae Liliaceae Asphodelaceae Magnoliaceae Asteraceae Malvaceae Betulaceae Moraceae Boraginaceae Myrtaceae Brassicaceae Oleaceae Bromeliaceae Orchidaceae Cactaceae Orobanchaceae Campanulaceae Pinaceae Caprifoliaceae Plantaginaceae Caryophyllaceae Poaceae Convolvulaceae Polygonaceae Cucurbitaceae Ranunculaceae Cupressaceae Rosaceae Cyperaceae Rubiaceae Equisetaceae Rutaceae Ericaceae Salicaceae Euphorbiaceae Scrophulariaceae -

Narcissus Bulbocodium
Report under the Article 17 of the Habitats Directive European Environment Period 2007-2012 Agency European Topic Centre on Biological Diversity Narcissus bulbocodium Annex V Priority No Species group Vascular plants Regions Atlantic, Mediterranean Narcissus bulbocodium is a perennial bulbous plant found across most of the Iberian Peninsula, except in the Pyrenees and on the Mediterranean coast, in the southwest of France and in Morocco. It is relatively common and it can be found in large scale of habitats like grasslands, heathlands, shrubs or forests. It is included in the Annex V of the Habitat Directive, as in past it was collected for commercial purposes. The species is listed as Least Concern (LC) in the IUCN European Red List. The conservation status was assessed as "Favourable" in both Atlantic and Mediterranean regions, but the status and trend of population are unknown and the assessment is only based on information provided for range, habitat of species and future prospects. In the Atlantic region the status is "Unfavourable Inadequate" in France, where the population size and range are declining and species is threatened by urbanization. No major threats are known in Portugal and Spain. Changes in overall conservation status between 2001-06 and 2007-12 report are mostly caused by different methodical approach and better data rather than real change in conservation status. Better data required from Spain. Page 1 Species: Narcissus bulbocodium Report under the Article 17 of the Habitats Directive Assessment of conservation -

Narcissus New for 2019
DRYAD NURSERY - SUMMER BULB LIST 2019 TEL 01423 358791 www.dryad-home.co.uk Email [email protected] Welcome to my 2019 list. I am very happy to be able to offer a particularly good number of famous Irish breeder Brian Duncan's very desirable new miniatures again. These are in very limited numbers, so you will have to be very quick! I recommend you email in your order as soon as possible. If you wish to telephone, I will note the time of your request and slot it into the appropriate place among the email requests. You can see better photos of the plants on my website. I hope you enjoy browsing, and look forward to hearing from you. PLEASE NOTE – MOST ITEMS ARE IN VERY LIMITED NUMBERS, SO PLEASE APPLY EARLY, LAST DATE FOR ORDERS END OF JULY 2019 ALL ITEMS OFFERED SUBJECT TO SUCCESSFUL HARVEST. UK and EU orders will be sent as soon as I can. Orders to outside the EU will all be sent together end of July/ August, as they will all need to be inspected together by FERA before dispatch. Snowdrops will be damp-packed for posting. All plants are flowering siZe, unless otherwise specified. POSTAGE AND PACKING- -£5-50 per order. I am asking for less than the actual cost of delivery and packaging. If your order can be sent as Large Letter, I will refund any extra postage. Parcels will be sent by first class post, recorded delivery, so will need to be signed for. Orders can be collected from my home address if you are in the area.