Annual Report of Form 20-F 2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Iconic Inclusive Growth Initiatives, Earmarked by B4IG Members
Iconic inclusive growth initiatives, earmarked by B4IG members 22/08/2019 The following are among the more than fifty initiatives earmarked by B4IG coalition members towards the platform. Some of these projects will be scaled, expanded or replicated through the B4IG incubator; others will be part of a B4IG innovation hub of inclusive business models that can be shared and emulated by other companies. These examples demonstrate the experience and resources that member companies bring to B4IG. More broadly, they show concretely the role that business can play in addressing inequalities of opportunity, gender and territory, and building truly inclusive societies. Company Name: Accenture Initiative Name: Inclusive Future of Work Inclusive Growth Lever: Equal Opportunity Countries Active: France, Ireland, Spain, United Arab Emirates, UK, US Description: Accenture’s Inclusive Future of Work initiative aims to help mid-career employees in routine roles transition to automation-resilient work. The Inclusive Future of Work Launchpad is geographically focused, bringing together key stakeholders from local business, government and non- profit sectors to identify and scale innovative products and services that address the obstacles to career change commonly encountered by experienced workers in less complex roles. The Launchpad has reached 300 people to date and is applying to the B4IG Incubator to expand its international network of organizations and experts involved, to access further funding to take successful models to scale, and to provide additional services to help workers equipped with new skills thrive in the digital economy. Company Name: Agropur Initiative Name: 1,000 First Days of Life Total Project Funding: 2 million CAD Inclusive Growth Lever: Equal Opportunity Countries Active: Canada Description: Agropur and Olo Foundation share a common goal: to support families in need to ensure babies are born healthy and to promote the development of sound eating habits in early childhood. -

Order 1 Purchase 2 Sale
Form to be sent to: ORDER 1 BNP Paribas Securities Services AIRBUS GROUP Securities Department Les Grands Moulins de Pantin 9, rue du Débarcadère 93761 PANTIN CEDEX - FRANCE 2 PURCHASE SALE Tel. : +33 1 57 43 35 00 Fax: +33 1 57 43 01 54 Confirmation of the order dated .… / … / ……. made by phone 3 DD/MM/YYYY I the undersigned, Mr. / Mrs. / Ms. Last name First name(s) (Strike out as appropriate) (For legal persons: name of signatory) (For legal persons: first name of signatory) Company name SIREN (For legal persons) Date and place of at Phone birth (DD/MM/YYYY) (Mandatory) Account number Residing at Town Post code Country Tax address (if different only) give instructions to BNP Paribas Securities Services to transmit the following order: Stock AIRBUS GROUP ISIN Code NL0000235190 Number of shares (In words) (In figures) i1 Type of order At market price Limit order at ________________________ EUR (Specify the maximum purchase price or minimum sale price) Validity of order (maximum end of month): __________________________ Account details to be credited by wire: Name of the account holder Bank’s name Bank address Country Account currency In France: Code Banque Code Guichet Numéro de compte Clé RIB In other countries4: BIC Code IBAN ABA Code BSB Code Bank Code Branch Code Bank account For payment to an account using an IBAN / BIC code, please specify the payment currency in which the account is held: ……………………………… Documents to be supplied for a sale order 5: a Bank Account identity (RIB), Postal Account identity (RIP), Savings Account identity (RICE) or IBAN number for payment by transfer of the proceeds of the sale of shares, after deduction of brokerage fees, taxes and commissions. -
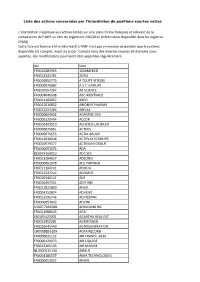
Liste Des Actions Concernées Par L'interdiction De Positions Courtes Nettes
Liste des actions concernées par l'interdiction de positions courtes nettes L’interdiction s’applique aux actions listées sur une plate-forme française et relevant de la compétence de l’AMF au titre du règlement 236/2012 (information disponible dans les registres ESMA). Cette liste est fournie à titre informatif. L'AMF n'est pas en mesure de garantir que le contenu disponible est complet, exact ou à jour. Compte tenu des diverses sources de données sous- jacentes, des modifications pourraient être apportées régulièrement. Isin Nom FR0010285965 1000MERCIS FR0013341781 2CRSI FR0010050773 A TOUTE VITESSE FR0000076887 A.S.T. GROUPE FR0010557264 AB SCIENCE FR0004040608 ABC ARBITRAGE FR0013185857 ABEO FR0012616852 ABIONYX PHARMA FR0012333284 ABIVAX FR0000064602 ACANTHE DEV. FR0000120404 ACCOR FR0010493510 ACHETER-LOUER.FR FR0000076861 ACTEOS FR0000076655 ACTIA GROUP FR0011038348 ACTIPLAY (GROUPE) FR0010979377 ACTIVIUM GROUP FR0000053076 ADA BE0974269012 ADC SIIC FR0013284627 ADEUNIS FR0000062978 ADL PARTNER FR0011184241 ADOCIA FR0013247244 ADOMOS FR0010340141 ADP FR0010457531 ADTHINK FR0012821890 ADUX FR0004152874 ADVENIS FR0013296746 ADVICENNE FR0000053043 ADVINI US00774B2088 AERKOMM INC FR0011908045 AG3I ES0105422002 AGARTHA REAL EST FR0013452281 AGRIPOWER FR0010641449 AGROGENERATION CH0008853209 AGTA RECORD FR0000031122 AIR FRANCE -KLM FR0000120073 AIR LIQUIDE FR0013285103 AIR MARINE NL0000235190 AIRBUS FR0004180537 AKKA TECHNOLOGIES FR0000053027 AKWEL FR0000060402 ALBIOMA FR0013258662 ALD FR0000054652 ALES GROUPE FR0000053324 ALPES (COMPAGNIE) -

Propranolol-Mediated Attenuation of MMP-9 Excretion in Infants with Hemangiomas
Supplementary Online Content Thaivalappil S, Bauman N, Saieg A, Movius E, Brown KJ, Preciado D. Propranolol-mediated attenuation of MMP-9 excretion in infants with hemangiomas. JAMA Otolaryngol Head Neck Surg. doi:10.1001/jamaoto.2013.4773 eTable. List of All of the Proteins Identified by Proteomics This supplementary material has been provided by the authors to give readers additional information about their work. © 2013 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 10/01/2021 eTable. List of All of the Proteins Identified by Proteomics Protein Name Prop 12 mo/4 Pred 12 mo/4 Δ Prop to Pred mo mo Myeloperoxidase OS=Homo sapiens GN=MPO 26.00 143.00 ‐117.00 Lactotransferrin OS=Homo sapiens GN=LTF 114.00 205.50 ‐91.50 Matrix metalloproteinase‐9 OS=Homo sapiens GN=MMP9 5.00 36.00 ‐31.00 Neutrophil elastase OS=Homo sapiens GN=ELANE 24.00 48.00 ‐24.00 Bleomycin hydrolase OS=Homo sapiens GN=BLMH 3.00 25.00 ‐22.00 CAP7_HUMAN Azurocidin OS=Homo sapiens GN=AZU1 PE=1 SV=3 4.00 26.00 ‐22.00 S10A8_HUMAN Protein S100‐A8 OS=Homo sapiens GN=S100A8 PE=1 14.67 30.50 ‐15.83 SV=1 IL1F9_HUMAN Interleukin‐1 family member 9 OS=Homo sapiens 1.00 15.00 ‐14.00 GN=IL1F9 PE=1 SV=1 MUC5B_HUMAN Mucin‐5B OS=Homo sapiens GN=MUC5B PE=1 SV=3 2.00 14.00 ‐12.00 MUC4_HUMAN Mucin‐4 OS=Homo sapiens GN=MUC4 PE=1 SV=3 1.00 12.00 ‐11.00 HRG_HUMAN Histidine‐rich glycoprotein OS=Homo sapiens GN=HRG 1.00 12.00 ‐11.00 PE=1 SV=1 TKT_HUMAN Transketolase OS=Homo sapiens GN=TKT PE=1 SV=3 17.00 28.00 ‐11.00 CATG_HUMAN Cathepsin G OS=Homo -

Airbus Corporate Jets Wins First Six ACJ Twotwenty Orders
Airbus Corporate Jets wins first six ACJ TwoTwenty orders #ACJTwoTwenty #ACJ #COMLUX Toulouse, 6 October 2020 - Airbus Corporate Jets has won its first orders for the ACJ TwoTwenty totalling six aircraft following its launch. While Comlux has revealed an order for two aircraft, four further jets were ordered by undisclosed customers. Entry into service of the first ACJ TwoTwenty by Comlux Aviation is targeted for early 2023. The new ACJ TwoTwenty will feature a high end VIP cabin interior, supported by a flexible cabin catalogue, from which Comlux has selected the business and guest lounge as well as a private entertainment space and a private suite, including a bathroom. The cabin, set to “Reimagine your place in the sky...” will be equipped with large full lie flat seats, a US-king size bed, a standing rainshower, a humidifying system for well-being on board and leading edge connectivity. “We are proud to be the launch customer of the Airbus’ newest family member, the ACJ TwoTwenty and the selected partner to outfit the cabin in our completion center in Indianapolis. We have worked jointly with ACJ and shared our long experience in operating and completing all types of aircraft, to allow the new Bizjet to offer more comfort and the latest cabin innovations available in the industry, “ said Richard Gaona, Executive Chairman & CEO Comlux. “Thanks to the unique combination of intercontinental range, comfort, extra space and second-to-none economics, we are convinced the aircraft will be a winner in the business aviation market.” “We are honoured to see our longstanding client Comlux becoming the launch customer of our new ACJ TwoTwenty as well as our cabin completion partner on the programme, “ said Benoit Defforge, President ACJ. -

Combined Ordinary and Extraordinary Shareholders’ Meeting of Danone Thursday 29 April 2021, at 2:30 P.M
NOTICE OF MEETING COMBINED ORDINARY AND EXTRAORDINARY SHAREHOLDERS’ MEETING OF DANONE THURSDAY 29 APRIL 2021, AT 2:30 P.M. DANONE Registered Office: 17, boulevard Haussmann, 75009 Paris – France A French Société Anonyme with a share capital of €171,657,400 552 032 534 RCS Paris CONTENTS CHAIRMAN’S MESSAGE 3 KEY FIGURES 2020 4 SUMMARY OF THE COMPANY’S SITUATION DURING THE LAST FISCAL YEAR 5 FINANCIAL RESULTS OF THE COMPANY DURING THE LAST FIVE FISCAL YEARS AND OTHER SIGNIFICANT INFORMATION 11 AGENDA OF THE SHAREHOLDERS’ MEETING 12 HOW TO PARTICIPATE IN THE SHAREHOLDERS’ MEETING? 13 HOW TO COMPLETE YOUR VOTING FORM? 16 YOUR BOARD OF DIRECTORS IN 2020 18 APPOINTMENT AND RENEWALS OF TERMS OF OFFICE PROPOSED TO THE SHAREHOLDERS’ MEETING 19 REPORT FROM THE BOARD OF DIRECTORS AND RESOLUTIONS SUBMITTED TO THE SHAREHOLDERS’ MEETING 24 SPECIAL REPORTS OF THE STATUTORY AUDITORS 46 TRANSFER OF SECURITIES INTO A DIRECT REGISTERED ACCOUNT 54 REQUEST FOR ADDITIONAL INFORMATION 55 WARNING – COVID-19 IN THE CONTEXT OF THE COVID-19 HEALTH CRISIS, THE SHAREHOLDERS’ MEETING OF 29 APRIL 2021 WILL BE HELD BEHIND CLOSED DOORS, WITHOUT THE SHAREHOLDERS AND OTHER PERSONS ENTITLED TO ATTEND BEING PHYSICALLY PRESENT. SHAREHOLDERS ARE INVITED TO CAST THEIR VOTE REMOTELY, PRIOR TO THE SHAREHOLDERS’ MEETING. YOU WILL FIND MORE INFORMATION IN THIS DOCUMENT. CHAIRMAN’S MESSAGE Ladies and Gentlemen, dear Shareholders, I am pleased to inform you that Danone’s Shareholders Meeting will be held on Thursday, 29 April 2021 at 2:30 pm. I regret not being able to welcome you to this Shareholders’ Meeting that I will have the honor to chair for the first time. -

2012-13 Annual Report of Private Giving
MAKING THE EXTRAORDINARY POSSIBLE 2012–13 ANNUAL REPORT OF PRIVATE GIVING 2 0 1 2–13 ANNUAL REPORT OF PRIVATE GIVING “Whether you’ve been a donor to UMaine for years or CONTENTS have just made your first gift, I thank you for your Letter from President Paul Ferguson 2 Fundraising Partners 4 thoughtfulness and invite you to join us in a journey Letter from Jeffery Mills and Eric Rolfson 4 that promises ‘Blue Skies ahead.’ ” President Paul W. Ferguson M A K I N G T H E Campaign Maine at a Glance 6 EXTRAORDINARY 2013 Endowments/Holdings 8 Ways of Giving 38 POSSIBLE Giving Societies 40 2013 Donors 42 BLUE SKIES AHEAD SINCE GRACE, JENNY AND I a common theme: making life better student access, it is donors like you arrived at UMaine just over two years for others — specifically for our who hold the real keys to the ago, we have truly enjoyed our students and the state we serve. While University of Maine’s future level interactions with many alumni and I’ve enjoyed many high points in my of excellence. friends who genuinely care about this personal and professional life, nothing remarkable university. Events like the surpasses the sense of reward and Unrestricted gifts that provide us the Stillwater Society dinner and the accomplishment that accompanies maximum flexibility to move forward Charles F. Allen Legacy Society assisting others to fulfill their are one of these keys. We also are luncheon have allowed us to meet and potential. counting on benefactors to champion thank hundreds of donors. -

Single-Cell RNA Sequencing Demonstrates the Molecular and Cellular Reprogramming of Metastatic Lung Adenocarcinoma
ARTICLE https://doi.org/10.1038/s41467-020-16164-1 OPEN Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma Nayoung Kim 1,2,3,13, Hong Kwan Kim4,13, Kyungjong Lee 5,13, Yourae Hong 1,6, Jong Ho Cho4, Jung Won Choi7, Jung-Il Lee7, Yeon-Lim Suh8,BoMiKu9, Hye Hyeon Eum 1,2,3, Soyean Choi 1, Yoon-La Choi6,10,11, Je-Gun Joung1, Woong-Yang Park 1,2,6, Hyun Ae Jung12, Jong-Mu Sun12, Se-Hoon Lee12, ✉ ✉ Jin Seok Ahn12, Keunchil Park12, Myung-Ju Ahn 12 & Hae-Ock Lee 1,2,3,6 1234567890():,; Advanced metastatic cancer poses utmost clinical challenges and may present molecular and cellular features distinct from an early-stage cancer. Herein, we present single-cell tran- scriptome profiling of metastatic lung adenocarcinoma, the most prevalent histological lung cancer type diagnosed at stage IV in over 40% of all cases. From 208,506 cells populating the normal tissues or early to metastatic stage cancer in 44 patients, we identify a cancer cell subtype deviating from the normal differentiation trajectory and dominating the metastatic stage. In all stages, the stromal and immune cell dynamics reveal ontological and functional changes that create a pro-tumoral and immunosuppressive microenvironment. Normal resident myeloid cell populations are gradually replaced with monocyte-derived macrophages and dendritic cells, along with T-cell exhaustion. This extensive single-cell analysis enhances our understanding of molecular and cellular dynamics in metastatic lung cancer and reveals potential diagnostic and therapeutic targets in cancer-microenvironment interactions. 1 Samsung Genome Institute, Samsung Medical Center, Seoul 06351, Korea. -

BIG CHALLENGES BIG SOLUTIONS 2121 Pennsylvania Avenue, NW Washington, DC 20433 USA IFC FINANCIALS and PROJECTS 2014
2014 2014 IFC Financials and Projects 2014 CREATING OPPORTUNITY WHERE IT’S NEEDED MOST BIG CHALLENGES BIG SOLUTIONS 2121 Pennsylvania Avenue, NW Washington, DC 20433 USA IFC FINANCIALS AND PROJECTS 2014 202 473 3800 ifc.org Big Challenges. Solutions. 2014 2014 TABLE OF CONTENTS MANAGEMENT’S DISCUSSION AND ANALYSIS 2 Executive Summary 2 Client Services 5 Liquid Assets 11 Funding Resources 11 Risk Management 13 Critical Accounting Policies 18 Results of Operations 20 Governance and Control 26 CONSOLIDATED FINANCIAL STATEMENTS AND INTERNAL CONTROL REPORTS 30 Management’s Report Regarding Effectiveness of Internal Control over External Financial Reporting 30 Auditors’ Report on Management’s Assertion on Effectiveness of Internal Control over External Financial Reporting 32 Consolidated Balance Sheets 34 Consolidated Income Statements 35 Consolidated Statements of Comprehensive Income 36 Consolidated Statements of Changes in Capital 37 Consolidated Statements of Cash Flows 39 Consolidated Statement of Capital Stock and Voting Power 41 Notes to Consolidated Financial Statements 42 Independent Auditors’ Report 100 PROJECT COMMITMENTS 103 INVESTMENT PORTFOLIO — CUMULATIVE GROSS COMMITMENTS BY REGION 124 NOTES AND DEFINITIONS 128 IFC Financials and Projects 2014 2 MANAGEMENT’S DISCUSSION AND ANALYSIS I. EXECUTIVE SUMMARY and other derivative instruments. The Management’s Discussion and Analysis contains forward looking statements which may be International Finance Corporation (IFC or the Corporation) is the identified by such terms as “anticipates,” “believes,” “expects,” largest global development institution focused on the private sec- “intends,” “plans” or words of similar meaning. Such statements tor in developing countries. Established in 1956, IFC is owned by involve a number of assumptions and estimates that are based on 184 member countries, a group that collectively determines its poli- current expectations, which are subject to risks and uncertainties cies. -

The Year of Investment, Innovation and Implementation
2017 the year of investment, innovation and implementation 2017 annual report healthcare businesswomen’s association table of contents LETTER FROM CHAIR 1 INVESTMENT 2017 BY THE NUMBERS 2 MEMBERSHIP 2 CHAPTER BREAKDOWN 2 40 YEARS OF MILESTONES 2 MEMBER/VOLUNTEER SURVEY RESULTS 3 FINANCIALS 4 INNOVATION AND IMPLEMENTATION 2017 FLAGSHIP EVENTS 7 WOMAN OF THE YEAR 8 LUMINARIES 10 RISING STARS 11 ANNUAL CONFERENCE 13 ACE AWARDS 14 THE GENDER PARITY COLLABORATIVE CLICK HERE HBA “NOW” THE NEW OPERATING MODEL CLICK HERE IN APPRECIATION—WE COULDN’T DO IT WITHOUT YOU 2017 CORPORATE PARTNERS 15 2017 SPONSORS 16 2017 MEDIA PARTNERS 17 HBA ADVISORY BOARD 18 HBA BOARD OF DIRECTORS 19 LETTER FROM CEO LAURIE COOKE 20 HBA 2017 II annual report letter from the chair In my long career as a healthcare executive, I have seen firsthand the benefits of diversity in leadership time and again. From gender to ethnicity to age and other factors, the best engagement and the most powerful results always arise when there is a diversity of perspectives in the room. This is why I joined the HBA board. This year marks the 40th anniversary of the organization’s founding. And though I am a relatively new member, I quickly became a believer in the HBA’s rich history and deep commitment to both the advancement of women individually, and to the achievement of gender parity in healthcare overall. This is also why I am proud of the HBA’s aggressive—but achievable—strategic plan to move the needle on gender parity through partnership by 2020. -

Business Combinations and Changes in Ownership Interests
25263 bd IFRS3 IAS27:25263 IFRS3/IAS27 bd 4/7/08 10:02 Page a Business combinations and changes in ownership interests A guide to the revised IFRS 3 and IAS 27 Audit.Tax.Consulting.Financial Advisory. 25263 bd IFRS3 IAS27:25263 IFRS3/IAS27 bd 4/7/08 10:02 Page b Contacts Global IFRS leadership team IFRS global office Global IFRS leader Ken Wild [email protected] IFRS centres of excellence Americas Robert Uhl [email protected] Asia Pacific Hong Kong Melbourne Stephen Taylor Bruce Porter [email protected] [email protected] Europe-Africa Copenhagen Johannesburg London Paris Jan Peter Larsen Graeme Berry Veronica Poole Laurence Rivat [email protected] [email protected] [email protected] [email protected] Deloitte’s www.iasplus.com website provides comprehensive information about international financial reporting in general and IASB activities in particular. Unique features include: • daily news about financial reporting globally. • summaries of all Standards, Interpretations and proposals. • many IFRS-related publications available for download. • model IFRS financial statements and checklists. • an electronic library of several hundred IFRS resources. • all Deloitte Touche Tohmatsu comment letters to the IASB. • links to several hundred international accounting websites. • e-learning modules for each IAS and IFRS – at no charge. • complete history of adoption of IFRSs in Europe and information about adoptions of IFRSs elsewhere around the world. • updates on developments in national accounting standards. 25263 bd IFRS3 IAS27:25263 IFRS3/IAS27 bd 4/7/08 10:02 Page c Contents 1. Introduction 1 1.1 Summary of major changes 1 1.2 Convergence of IFRSs and US GAAP 3 2. -

REFERENCE DOCUMENT Including the Sustainable Development Report Contents
2015 REFERENCE DOCUMENT Including the Sustainable Development Report Contents Key Figures 2 1 Management Report 9 4 Financial statements 199 History of the Air Liquide Group 10 Consolidated fi nancial statements 201 Activities and risk factors 15 Statutory accounts of the parent company 275 2015 Performance 32 Investment cycle and fi nancing strategy 45 Innovation 53 Strategy and outlook 62 5 Annual General Meeting 2016 297 Board of Directors’ Report on the resolutions presented to the 2016 Combined Shareholders’ 2 2015 Corporate Social Responsibility Meeting 298 and Sustainable Development Report 65 Resolutions presented for the approval of the Combined Shareholders’ Meeting – Introduction 66 May 12, 2016 307 Our 2015 Social and Environmental Contribution 67 Statutory Auditors’ Reports 327 Environmental, social and governance (ESG) report 69 Reporting methodology 113 Independent verifi er’s report 115 Appendix 118 6 Additional information 339 Share capital 340 General information 346 3 Corporate governance 119 Trade payables 356 Factors that may have an impact in the event Management and control 120 of a takeover bid 357 Report from the Chairman of the Board of Directors 123 Person responsible for the Reference Document 359 Remuneration of the Executive Offi cers and Directors Cross-reference table for the Reference Document 360 of L’Air Liquide S.A. 147 Cross-reference table for the Annual Financial Report 364 Statutory Auditors’ Report 174 Cross-reference table for the Management Report 365 Transactions involving Company shares performed