Atlas of Advance Endoaortic Spine Surgery.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Total Arch Replacement for Aortic Arch Aneurysm with Coexisting Middle Aortic Syndrome
CASE REPORT – OPEN ACCESS International Journal of Surgery Case Reports 54 (2019) 79–82 Contents lists available at ScienceDirect International Journal of Surgery Case Reports journa l homepage: www.casereports.com Total arch replacement for aortic arch aneurysm with coexisting middle aortic syndrome ∗ Zaiqiang Yu, Masahito Minakawa , Norihiro Kondo, Kazuyuki Daitoku, Ikuo Fukuda Department of Thoracic and Cardiovascular Surgery, Hirosaki University Graduate School of Medicine, Aomori, Japan a r a t i b s c t l e i n f o r a c t Article history: Introduction: Middle aortic syndrome (MAS) combined with thoracic aortic aneurysm (TAA) is a rare vas- Received 20 September 2018 cular disease. One stage open surgery to treat this condition, becomes a challenge for our cardiovascular Received in revised form surgery. 15 November 2018 Presentation of case: A 69-year-old man presented with a saccular type aortic arch aneurysm, shaggy Accepted 19 November 2018 aorta and severe atherosclerotic stenosis of the thoracoabdominal aorta with middle aortic syndrome and Available online 24 November 2018 aberrant right subclavian artery, renovascular hypertension, renal dysfunction, and intermittent claudi- cation of both legs. Total arch replacement procedure was performed under a cardiopulmonary bypass Keywords: using aortic inflow from the right axillary artery and a femoro-femoral crossover bypass graft to avoid Aortic arch aneurysm malperfusion of the lower body. Before weaning from the cardiopulmonary bypass, we established an Middle aortic syndrome Axillary-femoral artery bypass extra-anatomical bypass from the ascending aortic graft to the femoro-femoral crossover bypass graft. Case report 3D-CT showed patency of bypass graft without any sign of stenosis postoperative. -

Abdominal Aortic Aneurysm (AAA)
Introduction NCEPOD operates under the umbrella of the National Patient Safety Agency (NPSA) as an independent confidential enquiry, whose main aim is to improve the quality and safety of patient care. Evidence is drawn from all sections of hospital activity in England, Wales, Northern Ireland, Guernsey, the Isle of Man and the Defence Sector, both NHS and private. We are very grateful to all those who take part as advisors, local reporters and as recipients of individual case reporting forms. I would also like to express my sincere thanks to our clinical co-ordinators and all the permanent staff of NCEPOD for the enormous amount of work and enthusiasm which they have put into the production of this report and without which we could not hope to perform such detailed analysis of, and comment upon, clinically-related hospital activity. Once again we have produced a summary report to accompany distribution of the detailed data both on CD ROM and also on the NCEPOD website, both of which allow major advances in the presentation of our data. Unlike traditional NCEPOD studies and in keeping with our new title of National Confidential Enquiry into Patient Outcome and Death, this is a cohort study looking at a specific area of clinical activity, namely, the management of abdominal aortic aneurysm (AAA). This was a fully representative sample of all patients admitted to hospital with an AAA during the study period, not just of those who died after operation, and thus provided us with good denominator data. There were 844 patients included in the study, 752 of which involved open operations, 53 of which involved endovascular repairs and 79 of which were patients who did not undergo operation but received palliative care. -

Open Surgical Repair of Abdominal Aortic Aneurysms Maintains a Pivotal Role in the Endovascular Era
346 Open Surgical Repair of Abdominal Aortic Aneurysms Maintains a Pivotal Role in the Endovascular Era Christopher D. Blackstock, MD, PhD1 Benjamin M. Jackson, MD, FACS1 1 Division of Vascular Surgery and Endovascular Therapy, University of Address for correspondence Christopher D. Blackstock, MD, PhD, Pennsylvania, Philadelphia, Pennsylvania Division of Vascular Surgery and Endovascular Therapy, Hospital of the University of Pennsylvania, 3400 Spruce Street, Maloney-4, Semin Intervent Radiol 2020;37:346–355 Philadelphia, PA 19104 (e-mail: [email protected]). Abstract Since the advent of endovascular aortic repair (EVAR) nearly three decades ago, there has been a paradigm shift in the treatment of the abdominal aortic aneurysm (AAA) to favor EVAR due to its reduced operative mortality, less invasive nature, and faster Keywords recovery times. However, more recently there has been an accumulation of data from ► abdominal aortic large meta-analyses and randomized clinical trials revealing that EVAR has no survival aneurysm benefit after approximately 2 years and is associated with substantially higher rates of ► open aortic surgery reintervention and aneurysm rupture in the long term. These findings call into question ► aortic repair the durability of EVAR compared with open aortic repair and emphasize the need for ► endovascular aortic surgeons to remain competent with open aortic surgery in the modern era. This article repair will provide comprehensive review of a large body of literature comparing endovas- ► -

Final Program
75TH ANNUAL MEETING ANNIVERSARY annual meeting CENTRAL SURGICAL ASSOCIATION FINAL PROGRAM March 15–17, 2018 Columbus, OH Hilton Columbus Downtown CSA_2018_FinalProgram.indd 1 3/6/18 8:49 PM THE CSA THANKS OUR INDUSTRY PARTNERS The Central Surgical Association would like to thank the following organizations for their marketing support of the 2018 Annual Meeting: Legally Mine USA | Gold Sponsor The Central Surgical Association would like to thank the following companies for their generous support as Exhibitors: Bard Davol Ethicon, Johnson & Johnson Gore & Associates Hitachi Healthcare Integra LifeSciences Legally Mine USA Teleflex, Inc. U.S. Army Healthcare A CSA_2018_FinalProgram.indd 2 3/6/18 8:49 PM SAVE THE DATE CSA 2019 Annual Meeting March 7-9, 2019 Innisbrook Palm Harbor, Florida 1 CSA_2018_FinalProgram.indd 1 3/6/18 8:49 PM TABLE OF CONTENTS 3 Special Notes 5 Officers and Councilors, Society Representatives, Committees 7 Educational Objectives 9 Schedule-at-a-Glance 13 Scientific Program 35 Abstracts 137 Best Paper by a New Member 140 New Members 141 Membership List 143 Past Presidents 147 Past Officers 151 Central Surgical Association Foundation Board of Directors and Committee Members 153 CSA Capital Campaign 156 Enrichment Awards 156 Turcotte Award 163 Past Annual Meeting Locations 2 CENTRAL SURGICAL ASSOCIATION CSA_2018_FinalProgram.indd 2 3/6/18 8:49 PM SPECIAL NOTES The Hilton Columbus Downtown Hotel will serve as the headquarters for the 2018 CSA Annual Meeting. Registration is located in the Bellows Foyer and is open: Wednesday 3:00pm – 6:00pm Thursday 7:00am – 4:30pm Friday 7:00am – 5:30pm Saturday 7:30am – 11:30am All scientific sessions will be held in the Bellows Ballroom DEF and a parallel session in the Robert King Room. -

April 2019 Vol. 27. No. 1
Journal of the Hong Kong College of Cardiology Editor-in-Chief David Chung-Wah Siu Co-Editors Chu-Pak Lau Bryan Ping-Yen Yan Editorial Board Raymond Hon-Wah Chan Cyrus R Kumana Ngai-Shing Mok Wai-Kwong Chan Suet-Ting Lau John E Sanderson Wai-Hong Chen Yuk-Kong Lau Brian Tomlinson Chun-Ho Cheng Tin-Chu Law Hung-Fat Tse Bernard Cheung Kathy Lai-Fun Lee Kai-Fat Tse Chung-Seung Chiang Stephen Wai-Luen Lee Tak-Ming Tse Moses S S Chow Maurice P Leung Siu-Hong Wan Wing-Hing Chow Sum-Kin Leung Kwok-Yiu Wong Katherine Fan Wai-Suen Leung Alexander Shou-Pang Wong Chi-Lai Ho Wing-Hung Leung Kam-Sang Woo Kau-Chung Ho Shu-Kin Li Cheuk-Man Yu David Sai-Wah Ho Archie Ying-Sui Lo Journal of the Hong Kong College of Cardiology (ISSN 1027-7811) is published bi-yearly by Medcom Limited, Flat E8, 10th Floor, Ka Ming Court, 688-690 Castle Peak Road, Cheung Sha Wan, Kowloon, Hong Kong, tel (852) 2578 3833, fax (852) 2578 3929, email: [email protected] Indexed in EMBASE/Excerpta Medica J HK Coll Cardiol, Vol 27 April 2019 i INSTRUCTION FOR AUTHORS The Journal of the Hong Kong College of Cardiology publishes peer-reviewed articles on all aspects of cardiovascular disease, including original clinical studies, review articles and experimental investigations. As official journal of the Hong Kong College of Cardiology, the journal publishes abstracts of reports to be presented at the Scientific Sessions of the College as well as reports of the College-sponsored conferences. -

Ischemic Colitis Complicating Major Vascular Surgery Scott R
Surg Clin N Am 87 (2007) 1099–1114 Ischemic Colitis Complicating Major Vascular Surgery Scott R. Steele, MD, FACS Department of Surgery, Madigan Army Medical Center, Fort Lewis, WA 98431, USA Ischemic colitis is a well-described complication of major vascular surgery, mostly following open abdominal aortic aneurysm (AAA) repair and endovascular aneurysm repair (EVAR), but also with aortoiliac surgery, aortic dissection, and thoracic aneurysm repair [1,2]. Although Boley and colleagues [3] described ischemia of the colon in 1963 as a revers- ible process secondary to vascular occlusion, the term was not coined until 1966, when Marston and colleagues [4] described its three stages of evolu- tion (transient ischemia, late ischemic stricture, and gangrene), along with the natural history of the disease. Furthermore, the development of colonic ischemia as a result of major vascular and aortic surgery has only been well described since the 1970s [5–9]. The original reports were scattered and mostly consisted of autopsy studies, depicting not only the high mortality associated with this condition but also, in retrospect, the relative lack of in- sight as to explanations for its onset and progression. Unfortunately, recent outcomes have not shown much improvement. Luckily, the overall inci- dence remains low, estimated at between 0.6% and 3.1%, with higher rates attributed to ruptured aneurysms, open repair, and emergent surgery [10]. Following the development of ischemic colitis, mortality has been reported to be as high as 67%, highlighting the need for rapidly identifying the com- mencement of symptoms and, perhaps more importantly, those patients at risk, in attempt to prevent its onset [10,11]. -

Abstract Book
January 24-28, 2015 51 The Society of Thoracic Surgeons st st Annual Meeting 51 Annual Meeting San Diego, California January 24-28, 2015 Abstract Book The Society of Thoracic Surgeons gratefully acknowledges the following companies for providing educational grants for the STS 51st Annual Meeting. This list is accurate as of December 11, 2014. STS Platinum Benefactor Provided $50,000 or above Abbott Vascular STS Gold Benefactor Provided $25,000-$49,999 Medtronic STS Silver Benefactors Provided $10,000-$24,999 Biomet Microfixation HeartWare St Jude Medical Thoratec Corporation GENERAL INFORMATION MISSION STATEMENT The mission of The Society of Thoracic Surgeons is to enhance the ability of cardiothoracic surgeons to provide the highest quality patient care through education, research, and advocacy. OVERALL MEETING OBJECTIVE The overall objective of this meeting is to provide a forum for all cardiothoracic surgeons and their teams to learn the most up-to-date information on research, surgical techniques, patient management, and social, ethical, and political issues in order to maintain the highest level of care for the cardiothoracic patient. STS CONTINUING MEDICAL EDUCATION (CME) MISSION STATEMENT The continuing medical education mission of The Societyof Thoracic Surgeons is to provide a forum for reporting results of scientific research and for updating information in the disciplines of cardiovascular, general thoracic and congenital heart surgery. The principal continuing education programs conducted by the Society include an annual scientific meeting, self-study programs, and other stand-alone meetings. The Annual Meeting is composed of peer-reviewed scientific abstracts, invited overview presentations, small group presentations, presentations on new technologies and video programs. -

Aortofemoral Bypass
Service: Vascular Aortofemoral bypass What is an aortofemoral bypass? An aortofemoral bypass for your aortoiliac arterial disease, or peripheral arterial disease (PAD), involves opening your abdomen (tummy) and your groins to bypass your diseased arteries with an artificial piece of artery (surgical graft). This is a major procedure. It is usually only considered if you are otherwise healthy and you cannot have an angioplasty (balloon stretch / stent). All patients go to ‘intensive care’ after open aortic surgery. There is therefore a risk that the operation may be cancelled on the day due to a lack of an ICU bed. The surgery is under general anaesthetic (asleep on a ventilator). The aorta is approached through an incision on your abdomen – this may be ‘up and down’ or ‘across’. You will also have an incision in one or both groins (to access the femoral arteries). Your specialist team will assess you, and your arteries on CT, to decide the most appropriate incision to use in you. The procedure 1. The small intestine is moved out of the way, either across into the right-hand side of the abdomen or up out of the abdomen into a bag. 2. The aorta is exposed, usually up to the arteries to your kidneys, and down to where it divides to supply your legs. 3. Incisions are made in one or both groins to access your femoral arteries. 4. A drug called Heparin is given to reduce risk of blood clots. 5. Clamps are applied to the aorta and the graft is sewn on. 6. The graft limbs are tunnelled to the groin and sewn to the femoral arteries. -

Temporal Trends and Management of Acute Aortic Occlusion: a 21 Year Experience
Eur J Vasc Endovasc Surg (2019) 58, 690e696 Temporal Trends and Management of Acute Aortic Occlusion: A 21 Year Experience Olivia Grip *, Anders Wanhainen, Martin Björck Department of Surgical Sciences, Vascular Surgery, Uppsala University, Uppsala, Sweden WHAT THIS PAPER ADDS Acute aortic occlusion is a rare, serious, and yet poorly investigated event. It can be caused by different aeti- ologies and be treated in many different ways. This investigation includes far more patients than all previous reports and can therefore report on time trends including long term survival with reporting of similar results for patient subgroups. Objectives: The aim was to study patients with acute aortic occlusion (AAO), a rare and life threatening event, in a population based cohort and the outcome of surgical treatment. Methods: The Swedish nationwide vascular database (Swedvasc) was used to identify cases, and the population registry to study long term survival. Variables associated with outcome were tested with the chi-square test and analysis of variance. Results: During the 21 year study period (1994e2014), 693 cases of surgical treatment for AAO were included, with a yearly incidence of 3.6 per million inhabitants. Mean Æ SD age was 69.9 Æ 11.2 years, 352 patients (50.8%) were women, and mean Æ SD length of follow up was 5.2 Æ 5.5 years. Most patients presented with bilateral acute limb ischaemia (596 patients, 86.0%). The aetiology of AAO was native artery thrombosis in 458 patients (66.1%), saddle embolus in 152 (21.9%), and occluded graft/stent/stent grafts in 83 (12.0%). -

CX-2018-3Rd-Announcement-A4 V7
Years of Looking Forward Faculty Krassi Ivancev, Hamburg, Germany Peter Schneider, Honolulu, USA Eddie Chaloner, London, UK Ralf Kolvenbach, Düsseldorf, Germany Andrea Agostinucci, Turin, Italy Heinz Jakob, Essen, Germany Catherine Shanahan, London, UK Sylvain Chastanet, Nice, France Peter Konstantiniuk, Graz, Austria Thomas Albrecht, Berlin, Germany Houman Jalaie, Aachen, Germany Cliff Shearman, Southampton, UK Fahd Chaudhry, Knoxville, USA Reinhard Kopp, Regensburg, Germany Jose Almeida, Miami, USA William Jeffcoate, Nottingham, UK Mitchell Silver, Columbus, USA Larysa Chernukha, Kiev, Ukraine Ali Kordzadeh, Broomfield, UK Klaus Amendt, Mannheim, Germany Robert Jones, Birmingham, UK Konstantinos Spanos, Larissa, Greece Stefano Chiarandini, Trieste, Italy Albert Kota, Vellore, India Michele Antonello, Padua, Italy William Jordan, Atlanta, USA Ian Spark, Adelaide, Australia Khamin Chinsakchai, Bangkok, Thailand Drosos Kotelis, Aachen, Germany Frank Arko, Charlotte, USA Lowell Kabnick, New York, USA Marlon Spreen, Amsterdam, Netherlands Che Lok Felix Chow, Hong Kong Ulrich Kugelmann, Gunzburg, Germany Jennifer Ash, Champaign, USA Andrea Kahlberg, Mila, Italy Benjamin Starnes, Seattle, USA Joannis Constantinides, London, UK Gaetano La Barbera, Palermo, Italy Mollie Atherton Meek, Little Rock, USA John Kakisis, Athens, Greece Martin Takes, Basel, Switzerland Ricardo Costantini, Pilar, Argentina Paul Lajos, New York, USA Efthymios Avgerinos, Pittsburgh, USA Evi Kalodiki, London, UK Hong-Yau Tan, Bedford Park, Australia Alexander Coupland, -
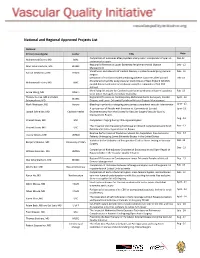
National and Regional Approved Projects List
National and Regional Approved Projects List National Primary Investigator Center Title Date Comparison of outcomes after popliteal artery repair: comparison of open vs. Dec-12 Mohammad Eslami, MD BMC endovascular repair Regional Differences in Lower Extremity Peripheral Arterial Disease Dec - 12 Marc Schermerhorn, MD BIDMC Management Variathoion and intensity of medical therapy in patients undergoing vascular Feb - 13 Randal DeMartino, MD DHMC surgery Derivation of risk factor model predicting adverse outcomes after Carotid Feb -13 Endarterectomy (CEA) using Vascular Study Group of New England (VSGNE) Mohammad Eslami, MD BMC carotid dataset and external validation using the remainder of SVS-PSO dataset Identifying risk factors for Cerebral reperfusion syndrome will permit patients Feb -13 Grace Wang, MD UPenn to be better managed and reduce morbidity. Thomas Curran, MD and Marc Regional Differences in Contemporary Abdominal Aortic Aneurysm, Carotid April - 13 BIDMC Schemerhorn, MD Disease, and Lower Extremity Peripheral Arterial Disease Management Mark Mewissen, MD Aurora Bleeding in patients undergoing percutaneous peripheral vascular intervention June - 13 A comparison of Results with Eversion vs. Conventional Carotid June -13 Joseph Schneider, MD Cadence Health Endarterectomy from the Society for Vascular Surgery Vascular Quality Improvement Project Aug - 13 Vincent Rowe, MD USC Completion Imaging During Infra-inguinal Bypass The Impact of Vein Harvesting Technique on Wound Complications and Graft Nov - 13 Vincent Rowe, MD USC Patency -

Connecting Hemosysis and Visceral Injury During Cardiovascular Surgery : Studies on the Causes, Effects, and Treatment of Hemolysis-Induced Organ Injury
Connecting hemosysis and visceral injury during cardiovascular surgery : studies on the causes, effects, and treatment of hemolysis-induced organ injury Citation for published version (APA): Vermeulen Windsant, I. C. (2012). Connecting hemosysis and visceral injury during cardiovascular surgery : studies on the causes, effects, and treatment of hemolysis-induced organ injury. Maastricht University. https://doi.org/10.26481/dis.20120928iv Document status and date: Published: 01/01/2012 DOI: 10.26481/dis.20120928iv Document Version: Publisher's PDF, also known as Version of record Please check the document version of this publication: • A submitted manuscript is the version of the article upon submission and before peer-review. There can be important differences between the submitted version and the official published version of record. People interested in the research are advised to contact the author for the final version of the publication, or visit the DOI to the publisher's website. • The final author version and the galley proof are versions of the publication after peer review. • The final published version features the final layout of the paper including the volume, issue and page numbers. Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal.