Proton Emission from Nuclei Due to Neutron Interactions 247
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Discovery of the Isotopes with Z<= 10
Discovery of the Isotopes with Z ≤ 10 M. Thoennessen∗ National Superconducting Cyclotron Laboratory and Department of Physics and Astronomy, Michigan State University, East Lansing, MI 48824, USA Abstract A total of 126 isotopes with Z ≤ 10 have been identified to date. The discovery of these isotopes which includes the observation of unbound nuclei, is discussed. For each isotope a brief summary of the first refereed publication, including the production and identification method, is presented. arXiv:1009.2737v1 [nucl-ex] 14 Sep 2010 ∗Corresponding author. Email address: [email protected] (M. Thoennessen) Preprint submitted to Atomic Data and Nuclear Data Tables May 29, 2018 Contents 1. Introduction . 2 2. Discovery of Isotopes with Z ≤ 10........................................................................ 2 2.1. Z=0 ........................................................................................... 3 2.2. Hydrogen . 5 2.3. Helium .......................................................................................... 7 2.4. Lithium ......................................................................................... 9 2.5. Beryllium . 11 2.6. Boron ........................................................................................... 13 2.7. Carbon.......................................................................................... 15 2.8. Nitrogen . 18 2.9. Oxygen.......................................................................................... 21 2.10. Fluorine . 24 2.11. Neon........................................................................................... -

Photofission Cross Sections of 238U and 235U from 5.0 Mev to 8.0 Mev Robert Andrew Anderl Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1972 Photofission cross sections of 238U and 235U from 5.0 MeV to 8.0 MeV Robert Andrew Anderl Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Nuclear Commons, and the Oil, Gas, and Energy Commons Recommended Citation Anderl, Robert Andrew, "Photofission cross sections of 238U and 235U from 5.0 MeV to 8.0 MeV " (1972). Retrospective Theses and Dissertations. 4715. https://lib.dr.iastate.edu/rtd/4715 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This dissertation was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction, 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity, 2. -
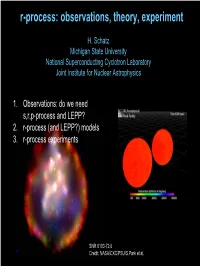
R-Process: Observations, Theory, Experiment
r-process: observations, theory, experiment H. Schatz Michigan State University National Superconducting Cyclotron Laboratory Joint Institute for Nuclear Astrophysics 1. Observations: do we need s,r,p-process and LEPP? 2. r-process (and LEPP?) models 3. r-process experiments SNR 0103-72.6 Credit: NASA/CXC/PSU/S.Park et al. Origin of the heavy elements in the solar system s-process: secondary • nuclei can be studied Æ reliable calculations • site identified • understood? Not quite … r-process: primary • most nuclei out of reach • site unknown p-process: secondary (except for νp-process) Æ Look for metal poor`stars (Pagel, Fig 6.8) To learn about the r-process Heavy elements in Metal Poor Halo Stars CS22892-052 (Sneden et al. 2003, Cowan) 2 1 + solar r CS 22892-052 ) H / X CS22892-052 ( g o red (K) giant oldl stars - formed before e located in halo Galaxyc was mixed n distance: 4.7 kpc theya preserve local d mass ~0.8 M_sol n pollutionu from individual b [Fe/H]= −3.0 nucleosynthesisa events [Dy/Fe]= +1.7 recall: element number[X/Y]=log(X/Y)-log(X/Y)solar What does it mean: for heavy r-process? For light r-process? • stellar abundances show r-process • process is not universal • process is universal • or second process exists (not visible in this star) Conclusions depend on s-process Look at residuals: Star – solar r Solar – s-process – p-process s-processSimmerer from Simmerer (Cowan et etal.) al. /Lodders (Cowan et al.) s-processTravaglio/Lodders from Travaglio et al. -0.50 -0.50 -1.00 -1.00 -1.50 -1.50 log e log e -2.00 -2.00 -2.50 -2.50 30 40 50 60 70 80 90 30 40 50 60 70 80 90 Element number Element number ÆÆNeedNeed reliable reliable s-process s-process (models (models and and nu nuclearclear data, data, incl. -

Uses of Isotopic Neutron Sources in Elemental Analysis Applications
EG0600081 3rd Conference on Nuclear & Particle Physics (NUPPAC 01) 20 - 24 Oct., 2001 Cairo, Egypt USES OF ISOTOPIC NEUTRON SOURCES IN ELEMENTAL ANALYSIS APPLICATIONS A. M. Hassan Department of Reactor Physics Reactors Division, Nuclear Research Centre, Atomic Energy Authority. Cairo-Egypt. ABSTRACT The extensive development and applications on the uses of isotopic neutron in the field of elemental analysis of complex samples are largely occurred within the past 30 years. Such sources are used extensively to measure instantaneously, simultaneously and nondestruclively, the major, minor and trace elements in different materials. The low residual activity, bulk sample analysis and high accuracy for short lived elements are improved. Also, the portable isotopic neutron sources, offer a wide range of industrial and field applications. In this talk, a review on the theoretical basis and design considerations of different facilities using several isotopic neutron sources for elemental analysis of different materials is given. INTRODUCTION In principle there are two ways to use neutrons for elemental and isotopic abundance analysis in samples. One is the neutron activation analysis which we call it the "off-line" where the neutron - induced radioactivity is observed after the end of irradiation. The other one we call it the "on-line" where the capture gamma-rays is observed during the neutron bombardment. Actually, the sequence of events occurring during the most common type of nuclear reaction used in this analysis namely the neutron capture or (n, gamma) reaction, is well known for the people working in this field. The neutron interacts with the target nucleus via a non-elastic collision, a compound nucleus forms in an excited state. -

Two-Proton Radioactivity 2
Two-proton radioactivity Bertram Blank ‡ and Marek P loszajczak † ‡ Centre d’Etudes Nucl´eaires de Bordeaux-Gradignan - Universit´eBordeaux I - CNRS/IN2P3, Chemin du Solarium, B.P. 120, 33175 Gradignan Cedex, France † Grand Acc´el´erateur National d’Ions Lourds (GANIL), CEA/DSM-CNRS/IN2P3, BP 55027, 14076 Caen Cedex 05, France Abstract. In the first part of this review, experimental results which lead to the discovery of two-proton radioactivity are examined. Beyond two-proton emission from nuclear ground states, we also discuss experimental studies of two-proton emission from excited states populated either by nuclear β decay or by inelastic reactions. In the second part, we review the modern theory of two-proton radioactivity. An outlook to future experimental studies and theoretical developments will conclude this review. PACS numbers: 23.50.+z, 21.10.Tg, 21.60.-n, 24.10.-i Submitted to: Rep. Prog. Phys. Version: 17 December 2013 arXiv:0709.3797v2 [nucl-ex] 23 Apr 2008 Two-proton radioactivity 2 1. Introduction Atomic nuclei are made of two distinct particles, the protons and the neutrons. These nucleons constitute more than 99.95% of the mass of an atom. In order to form a stable atomic nucleus, a subtle equilibrium between the number of protons and neutrons has to be respected. This condition is fulfilled for 259 different combinations of protons and neutrons. These nuclei can be found on Earth. In addition, 26 nuclei form a quasi stable configuration, i.e. they decay with a half-life comparable or longer than the age of the Earth and are therefore still present on Earth. -

Correlated Neutron Emission in Fission
Correlated neutron emission in fission S. Lemaire , P. Talou , T. Kawano , D. G. Madland and M. B. Chadwick ¡ Nuclear Physics group, Los Alamos National Laboratory, Los Alamos, NM, 87545 Abstract. We have implemented a Monte-Carlo simulation of the fission fragments statistical decay by sequential neutron emission. Within this approach, we calculate both the center-of-mass and laboratory prompt neutron energy spectra, the ¢ prompt neutron multiplicity distribution P ν £ , and the average total number of emitted neutrons as a function of the mass of ¢ the fission fragment ν¯ A £ . Two assumptions for partitioning the total available excitation energy among the light and heavy fragments are considered. Preliminary results are reported for the neutron-induced fission of 235U (at 0.53 MeV neutron energy) and for the spontaneous fission of 252Cf. INTRODUCTION Methodology In this work, we extend the Los Alamos model [1] by A Monte Carlo approach allows to follow in detail any implementing a Monte-Carlo simulation of the statistical reaction chain and to record the result in a history-type decay (Weisskopf-Ewing) of the fission fragments (FF) file, which basically mimics the results of an experiment. by sequential neutron emission. This approach leads to a We first sample the FF mass and charge distributions, much more detailed picture of the decay process and var- and pick a pair of light and heavy nuclei that will then de- ious physical quantities can then be assessed: the center- cay by emitting zero, one or several neutrons. This decay of-mass and laboratory prompt neutron energy spectrum sequence is governed by neutrons emission probabilities ¤ ¤ ¥ N en ¥ , the prompt neutron multiplicity distribution P n , at different temperatures of the compound nucleus and the average number of emitted neutrons as a function of by the energies of the emitted neutrons. -

Nuclear Glossary
NUCLEAR GLOSSARY A ABSORBED DOSE The amount of energy deposited in a unit weight of biological tissue. The units of absorbed dose are rad and gray. ALPHA DECAY Type of radioactive decay in which an alpha ( α) particle (two protons and two neutrons) is emitted from the nucleus of an atom. ALPHA (ααα) PARTICLE. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are a highly ionizing form of particle radiation, and have low penetration. Alpha particles are emitted by radioactive nuclei such as uranium or radium in a process known as alpha decay. Owing to their charge and large mass, alpha particles are easily absorbed by materials and can travel only a few centimetres in air. They can be absorbed by tissue paper or the outer layers of human skin (about 40 µm, equivalent to a few cells deep) and so are not generally dangerous to life unless the source is ingested or inhaled. Because of this high mass and strong absorption, however, if alpha radiation does enter the body through inhalation or ingestion, it is the most destructive form of ionizing radiation, and with large enough dosage, can cause all of the symptoms of radiation poisoning. It is estimated that chromosome damage from α particles is 100 times greater than that caused by an equivalent amount of other radiation. ANNUAL LIMIT ON The intake in to the body by inhalation, ingestion or through the skin of a INTAKE (ALI) given radionuclide in a year that would result in a committed dose equal to the relevant dose limit . -

Recent Developments in Radioactive Charged-Particle Emissions
Recent developments in radioactive charged-particle emissions and related phenomena Chong Qi, Roberto Liotta, Ramon Wyss Department of Physics, Royal Institute of Technology (KTH), SE-10691 Stockholm, Sweden October 19, 2018 Abstract The advent and intensive use of new detector technologies as well as radioactive ion beam facilities have opened up possibilities to investigate alpha, proton and cluster decays of highly unstable nuclei. This article provides a review of the current status of our understanding of clustering and the corresponding radioactive particle decay process in atomic nuclei. We put alpha decay in the context of charged-particle emissions which also include one- and two-proton emissions as well as heavy cluster decay. The experimental as well as the theoretical advances achieved recently in these fields are presented. Emphasis is given to the recent discoveries of charged-particle decays from proton-rich nuclei around the proton drip line. Those decay measurements have shown to provide an important probe for studying the structure of the nuclei involved. Developments on the theoretical side in nuclear many-body theories and supercomputing facilities have also made substantial progress, enabling one to study the nuclear clusterization and decays within a microscopic and consistent framework. We report on properties induced by the nuclear interaction acting in the nuclear medium, like the pairing interaction, which have been uncovered by studying the microscopic structure of clusters. The competition between cluster formations as compared to the corresponding alpha-particle formation are included. In the review we also describe the search for super-heavy nuclei connected by chains of alpha and other radioactive particle decays. -

Radioactive Decay & Decay Modes
CHAPTER 1 Radioactive Decay & Decay Modes Decay Series The terms ‘radioactive transmutation” and radioactive decay” are synonymous. Many radionuclides were found after the discovery of radioactivity in 1896. Their atomic mass and mass numbers were determined later, after the concept of isotopes had been established. The great variety of radionuclides present in thorium are listed in Table 1. Whereas thorium has only one isotope with a very long half-life (Th-232, uranium has two (U-238 and U-235), giving rise to one decay series for Th and two for U. To distin- guish the two decay series of U, they were named after long lived members: the uranium-radium series and the actinium series. The uranium-radium series includes the most important radium isotope (Ra-226) and the actinium series the most important actinium isotope (Ac-227). In all decay series, only α and β− decay are observed. With emission of an α particle (He- 4) the mass number decreases by 4 units, and the atomic number by 2 units (A’ = A - 4; Z’ = Z - 2). With emission β− particle the mass number does not change, but the atomic number increases by 1 unit (A’ = A; Z’ = Z + 1). By application of the displacement laws it can easily be deduced that all members of a certain decay series may differ from each other in their mass numbers only by multiples of 4 units. The mass number of Th-232 is 323, which can be written 4n (n=58). By variation of n, all possible mass numbers of the members of decay Engineering Aspects of Food Irradiation 1 Radioactive Decay series of Th-232 (thorium family) are obtained. -
Proton Decay at the Drip-Line Magic Number Z = 82, Corresponding to the Closure of a Major Nuclear Shell
NEWS AND VIEWS NUCLEAR PHYSICS-------------------------------- case since it has one proton more than the Proton decay at the drip-line magic number Z = 82, corresponding to the closure of a major nuclear shell. In Philip Woods fact the observed proton decay comes from an excited intruder state configura WHAT determines whether a nuclear ton decay half-life measurements can be tion formed by the promotion of a proton species exists? For nuclear scientists the used to explore nuclear shell structures in from below the Z=82 shell closure. This answer to this poser is that the species this twilight zone of nuclear existence. decay transition was used to provide should live long enough to be identified The proton drip-line sounds an inter unique information on the quantum mix and its properties studied. This still begs esting place to visit. So how do we get ing between normal and intruder state the fundamental scientific question of there? The answer is an old one: fusion. configurations in the daughter nucleus. what the ultimate boundaries to nuclear In this case, the fusion of heavy nuclei Following the serendipitous discovery existence are. Work by Davids et al. 1 at produces highly neutron-deficient com of nuclear proton decay4 from an excited Argonne National Laboratory in the pound nuclei, which rapidly de-excite by state in 1970, and the first example of United States has pinpointed the remotest boiling off particles and gamma-rays, leav ground-state proton decay5 in 1981, there border post to date, with the discovery of ing behind a plethora of highly unstable followed relative lulls in activity. -

Neutron Emission Spectrometry for Fusion Reactor Diagnosis
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1244 Neutron Emission Spectrometry for Fusion Reactor Diagnosis Method Development and Data Analysis JACOB ERIKSSON ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 ISBN 978-91-554-9217-5 UPPSALA urn:nbn:se:uu:diva-247994 2015 Dissertation presented at Uppsala University to be publicly examined in Polhemsalen, Ångströmlaboratoriet, Lägerhyddsvägen 1, Uppsala, Friday, 22 May 2015 at 09:15 for the degree of Doctor of Philosophy. The examination will be conducted in English. Faculty examiner: Dr Andreas Dinklage (Max-Planck-Institut für Plasmaphysik, Greifswald, Germany). Abstract Eriksson, J. 2015. Neutron Emission Spectrometry for Fusion Reactor Diagnosis. Method Development and Data Analysis. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1244. 92 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-554-9217-5. It is possible to obtain information about various properties of the fuel ions deuterium (D) and tritium (T) in a fusion plasma by measuring the neutron emission from the plasma. Neutrons are produced in fusion reactions between the fuel ions, which means that the intensity and energy spectrum of the emitted neutrons are related to the densities and velocity distributions of these ions. This thesis describes different methods for analyzing data from fusion neutron measurements. The main focus is on neutron spectrometry measurements, using data used collected at the tokamak fusion reactor JET in England. Several neutron spectrometers are installed at JET, including the time-of-flight spectrometer TOFOR and the magnetic proton recoil (MPRu) spectrometer. Part of the work is concerned with the calculation of neutron spectra from given fuel ion distributions. -

16. Fission and Fusion Particle and Nuclear Physics
16. Fission and Fusion Particle and Nuclear Physics Dr. Tina Potter Dr. Tina Potter 16. Fission and Fusion 1 In this section... Fission Reactors Fusion Nucleosynthesis Solar neutrinos Dr. Tina Potter 16. Fission and Fusion 2 Fission and Fusion Most stable form of matter at A~60 Fission occurs because the total Fusion occurs because the two Fission low A nuclei have too large a Coulomb repulsion energy of p's in a surface area for their volume. nucleus is reduced if the nucleus The surface area decreases Fusion splits into two smaller nuclei. when they amalgamate. The nuclear surface The Coulomb energy increases, energy increases but its influence in the process, is smaller. but its effect is smaller. Expect a large amount of energy released in the fission of a heavy nucleus into two medium-sized nuclei or in the fusion of two light nuclei into a single medium nucleus. a Z 2 (N − Z)2 SEMF B(A; Z) = a A − a A2=3 − c − a + δ(A) V S A1=3 A A Dr. Tina Potter 16. Fission and Fusion 3 Spontaneous Fission Expect spontaneous fission to occur if energy released E0 = B(A1; Z1) + B(A2; Z2) − B(A; Z) > 0 Assume nucleus divides as A , Z A1 Z1 A2 Z2 1 1 where = = y and = = 1 − y A, Z A Z A Z A2, Z2 2 2=3 2=3 2=3 Z 5=3 5=3 from SEMF E0 = aSA (1 − y − (1 − y) ) + aC (1 − y − (1 − y) ) A1=3 @E0 maximum energy released when @y = 0 @E 2 2 Z 2 5 5 0 = a A2=3(− y −1=3 + (1 − y)−1=3) + a (− y 2=3 + (1 − y)2=3) = 0 @y S 3 3 C A1=3 3 3 solution y = 1=2 ) Symmetric fission Z 2 2=3 max.