Purification of Aqueous, Protic Ionic Liquid by Distillation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Separation of Three Azeotropes by Extractive Distillation by An-I Yeh A
The separation of three azeotropes by extractive distillation by An-I Yeh A thesis submitted in partial fulfillment of the requirement for the degree of Master of Science in Chemical Engineering Montana State University © Copyright by An-I Yeh (1983) Abstract: Several different kinds of extractive distillation agents were investigated to affect the separation of three binary liquid mixtures, isopropyl ether - acetone, methyl acetate - methanol, and isopropyl ether - methyl ethyl ketone. Because of the small size of the extractive distillation column, relative volatilities were assumed constant and the Fenske equation was used to calculate the relative volatilities and the number of minimum theoretical plates. Dimethyl sulfoxide was found to be a good extractive distillation agent. Extractive distillation when employing a proper agent not only negated the azeotropes of the above mixtures, but also improved the efficiency of separation. This process could reverse the relative volatility of isopropyl ether and acetone. This reversion was also found in the system of methyl acetate and methanol when nitrobenzene was the agent. However, normal distillation curves were obtained for the system of isopropyl ether and methyl ethyl ketone undergoing extractive distillation. In the system of methyl acetate and methanol, the relative volatility decreased as the agents' carbon number increased when glycols were used as the agents. In addition, the oxygen number and the locations of hydroxyl groups in the glycols used were believed to affect the values of relative volatility. An appreciable amount of agent must be maintained in the column to affect separation. When dimethyl sulfoxide was an agent for the three systems studied, the relative volatility increased as the addition rate increased. -

Development of a Process for N-Butanol Recovery from Abe
937 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 74, 2019 The Italian Association of Chemical Engineering Online at www.cetjournal.it Guest Editors: Sauro Pierucci, Jiří Jaromír Klemeš, Laura Piazza Copyright © 2019, AIDIC Servizi S.r.l. ISBN 978-88-95608-71-6; ISSN 2283-9216 DOI: 10.3303/CET1974157 Development of a Process for N-Butanol Recovery from Abe Wastewater Streams by Membrane Technology * Marco Stoller , Marco Bravi, Paola Russo, Roberto Bubbico, Barbara Mazzarotta Sapienza University of Rome, Dept. of Chemical Materials Environmental Engineering, Via Eudossiana 18, 00184 Rome, Italy [email protected] The aceton-butyl-ethanolic fermentation process (ABE) is a biotechnological process that leads to the production of acetone, n-butanol and ethanol (ABE compounds) from glucose sources and amides by use of certain biomasses. The process was developed initially during the middle of the last century and suffers from decline due to the greater petrochemical production of products and the lowering of the costs of the sector. Nowadays, the ABE process is regaining great interest because the fraction with the highest concentration, i.e. n-butanol, is an excellent constituent for biofuels. The ABE process has been optimized over time to obtain maximum yields of n-butanol, but the problem of separating and concentrating the butanol in the outlet stream of the ABE process persists. To allow an adequate use, often distillation by use of more columns is required. Moreover, the contained biomasses and suspended solids, in high quantity, must be eliminated, leading to overall high treatment costs. This work will report the main idea and some preliminary experimental results for the development and application of a process based on membrane technologies, to separate and concentrate the butanol from ABE process streams to sensibly reduce the difficulty to perform a final distillation. -

Evaluation of Azeotropic Dehydration for the Preservation of Shrimp. James Edward Rutledge Louisiana State University and Agricultural & Mechanical College
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1969 Evaluation of Azeotropic Dehydration for the Preservation of Shrimp. James Edward Rutledge Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Rutledge, James Edward, "Evaluation of Azeotropic Dehydration for the Preservation of Shrimp." (1969). LSU Historical Dissertations and Theses. 1689. https://digitalcommons.lsu.edu/gradschool_disstheses/1689 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been 70-9089 microfilmed exactly as received RUTLEDGE, James Edward, 1941- EVALUATION OF AZEOTROPIC DEHYDRATION FOR THE PRESERVATION OF SHRIMP. The Louisiana State University and Agricultural and Mechanical College, PhJD., 1969 Food Technology University Microfilms, Inc., Ann Arbor, Michigan Evaluation of Azeotropic Dehydration for the Preservation of Shrimp A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Food Science and Technology by James Edward Rutledge B.S., Texas A&M University, 1963 M.S., Texas A&M University, 1966 August, 1969 ACKNOWLEDGMENT The author wishes to express his sincere appreciation to his major professor, Dr, Fred H. Hoskins, for the guidance which he supplied not only in respect to this dissertation but also in regard to the author’s graduate career at Louisiana State University, Gratitude is also extended to Dr. -
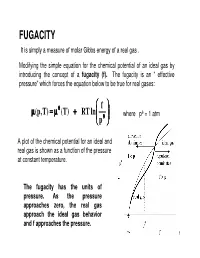
FUGACITY It Is Simply a Measure of Molar Gibbs Energy of a Real Gas
FUGACITY It is simply a measure of molar Gibbs energy of a real gas . Modifying the simple equation for the chemical potential of an ideal gas by introducing the concept of a fugacity (f). The fugacity is an “ effective pressure” which forces the equation below to be true for real gases: θθθ f µµµ ,p( T) === µµµ (T) +++ RT ln where pθ = 1 atm pθθθ A plot of the chemical potential for an ideal and real gas is shown as a function of the pressure at constant temperature. The fugacity has the units of pressure. As the pressure approaches zero, the real gas approach the ideal gas behavior and f approaches the pressure. 1 If fugacity is an “effective pressure” i.e, the pressure that gives the right value for the chemical potential of a real gas. So, the only way we can get a value for it and hence for µµµ is from the gas pressure. Thus we must find the relation between the effective pressure f and the measured pressure p. let f = φ p φ is defined as the fugacity coefficient. φφφ is the “fudge factor” that modifies the actual measured pressure to give the true chemical potential of the real gas. By introducing φ we have just put off finding f directly. Thus, now we have to find φ. Substituting for φφφ in the above equation gives: p µ=µ+(p,T)θ (T) RT ln + RT ln φ=µ (ideal gas) + RT ln φ pθ µµµ(p,T) −−− µµµ(ideal gas ) === RT ln φφφ This equation shows that the difference in chemical potential between the real and ideal gas lies in the term RT ln φφφ.φ This is the term due to molecular interaction effects. -

United States Patent 19 11 Patent Number: 5,364,534 Anselme Et Al
US005364534A United States Patent 19 11 Patent Number: 5,364,534 Anselme et al. 45 Date of Patent: Nov. 15, 1994 54 PROCESS AND APPARATUS FOR 4,872,991 10/1989 Kartels et al. ...................... 210/651 TREATING WASTE LIQUIDS 5,093,072 8/1991 Hitotsuyanagi et al. ........... 210/650 5,154,830 10/1992 Paul et al. ........................... 210/639 75 Inventors: Christophe Anselme, Le Vesinet; Isabelle Baudin, Nanterre, both of FOREIGN PATENT DOCUMENTS France 2628337 9/1989 France . 73 Assignee: Lyonnaise Des Eaux - Dumez, 4018994 1/1992 Japan ................................ 210/195.2 Nanterre, France Primary Examiner-Frank Spear (21) Appl. No.: 129,387 Assistant Examiner-Ana Fortuna Attorney, Agent, or Firm-Pollock, Vande Sande & 22 Filed: Sep. 30, 1993 Priddy (30) Foreign Application Priority Data 57 ABSTRACT Oct. 2, 1992 FR France ................................ 92 1699 Process for purifying and filtering fluids, especially 51l Int. Cl............................................... BOD 61/00 water, containing suspended contaminants and using 52 U.S. Cl. .................................... 210/650; 210/660; gravity separation means as well as membrane separa 210/800; 210/805; 210/195.1; 210/195.2; tion means, in a finishing stage, comprising the step of 210/257.2 introducing a pulverulent reagent into the fluid stream 58) Field of Search ............... 210/650, 639, 800, 790, to be treated downstream of the gravity separation and 210/195.1, 195.2, 295, 805, 900, 257.2, 660 upstream of the membrane separation, wherein said 56) References Cited pulverulent reagent is recycled from the purge of the U.S. PATENT DOCUMENTS membrane separation means to the upstream of the gravity separation means. -

A Case Study on Separation of IPA-Water Mixture by Extractive Distillation Using Aspen Plus
International Journal of Advanced Technology and Engineering Exploration, Vol 3(24) ISSN (Print): 2394-5443 ISSN (Online): 2394-7454 Research Article http://dx.doi.org/10.19101/IJATEE.2016.324004 A case study on separation of IPA-water mixture by extractive distillation using aspen plus Sarita Kalla, Sushant Upadhyaya*, Kailash Singh, Rajeev Kumar Dohare and Madhu Agarwal Department of Chemical Engineering, Malaviya National Institute of Technology, Jaipur, India ©2016 ACCENTS Abstract Extractive distillation is one of the popular methods being leveraged to separate isopropyl alcohol (IPA) from water present in waste stream in semi-conductor industries. In this context, the paper aims to carry out simulation study for separation of isopropyl alcohol-water azeotrope mixture using ethylene glycol as entrainer. In particular, temperature, pressure and concentration profile has been studied. Aspen plus® (version 8.8) has been used as simulation tool and optimum number of stages for the simulation turns out to be 42. The results show that top of the column contains IPA of 99.974 mol % purity and bottom product contains water-ethylene glycol mixture. Moreover, sensitivity analysis for different parameters and analysis of residue curve for ternary system have been also performed. Keywords IPA-water, Extractive distillation, Simulation, Entrainer. 1. Introduction 2. Process simulation Isopropyl alcohol is generally used as the cleaning 2.1 Thermodynamic model agent and solvent in chemical industries. Because of Thermodynamic model is used to describe phase its cleaning property it is also known as rubbing equilibria properties of the system. For the design of alcohol. IPA is soluble in water and it forms chemical separation operations the phase equilibrium azeotrope with water at temperature 80.3-80.4 0C. -

Review of Extractive Distillation. Process Design, Operation
Review of Extractive Distillation. Process design, operation optimization and control Vincent Gerbaud, Ivonne Rodríguez-Donis, Laszlo Hegely, Péter Láng, Ferenc Dénes, Xinqiang You To cite this version: Vincent Gerbaud, Ivonne Rodríguez-Donis, Laszlo Hegely, Péter Láng, Ferenc Dénes, et al.. Review of Extractive Distillation. Process design, operation optimization and control. Chemical Engineering Research and Design, Elsevier, 2019, 141, pp.229-271. 10.1016/j.cherd.2018.09.020. hal-02161920 HAL Id: hal-02161920 https://hal.archives-ouvertes.fr/hal-02161920 Submitted on 21 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Open Archive Toulouse Archive Ouverte OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible This is an author’s version published in: http://oatao.univ-toulouse.fr/239894 Official URL: https://doi.org/10.1016/j.cherd.2018.09.020 To cite this version: Gerbaud, Vincent and Rodríguez-Donis, Ivonne and Hegely, Laszlo and Láng, Péter and Dénes, Ferenc and You, Xinqiang Review of Extractive Distillation. Process design, operation optimization and control. (2018) Chemical Engineering Research and Design, 141. -

Computer Simulation of a Multicomponent, Multistage Batch Distillation Process
COMPUTER SIMULATION OF A MULTICOMPONENT, MULTISTAGE BATCH DISTILLATION PROCESS By BRUCE EARL BAUGHER /J Bachelor of Science in Chemical Engineering Oklahoma State University Stillwater, Oklahoma 1985 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE July, 1988 \\,~s~ ~ \'\~~ B~~~to' <:.o?. ·;)._, ~ r COMPUTER SIMULATION OF A MULTICOMPONENT, MULTISTAGE BATCH DISTILLATION PROCESS Thesis Approved: Thesis Advisor U.bJ ~~- ~MJYl~ Dean of the GradU~te College ABSTRACT The material and energy balance equations for a batch distillation column were derived and a computer simulation was developed to solve these equations. The solution follows a modified Newton-Raphson approach for inverting and solving the matrices of material and energy balance equations. The model is unique in that it has been designed to handle hypothetical hydrocarbon components. The simulation can handle columns up to 50 trays and systems of up to 100 components. The model has been used to simulate True Boiling Point (TBP) diagrams for a variety of crude oils. This simulation is also applicable to simple laboratory batch distillations. The model was designed to accurately combine data files to simulate actual crude blending procedures. The model will combine files and calculate the results quickly and easily. The simulation involves removing material from the column at steady-state. The removal fraction is made small enough to approach continuity. This simulation can be adapted for use on microcomputers, although it will require extensive computation time. ACKNOWLEDGMENTS It has been a great pleasure to work with my thesis advisor, Dr. -

United States Patent (10) Patent No.: US 9,586,922 B2 Wood Et Al
USOO9586922B2 (12) United States Patent (10) Patent No.: US 9,586,922 B2 Wood et al. (45) Date of Patent: Mar. 7, 2017 (54) METHODS FOR PURIFYING 9,388,150 B2 * 7/2016 Kim ..................... CO7D 307/48 5-(HALOMETHYL)FURFURAL 9,388,151 B2 7/2016 Browning et al. 2007/O161795 A1 7/2007 Cvak et al. 2009, 0234142 A1 9, 2009 Mascal (71) Applicant: MICROMIDAS, INC., West 2010, 0083565 A1 4, 2010 Gruter Sacramento, CA (US) 2010/0210745 A1 8/2010 McDaniel et al. 2011 O144359 A1 6, 2011 Heide et al. (72) Inventors: Alex B. Wood, Sacramento, CA (US); 2014/01 00378 A1 4/2014 Masuno et al. Shawn M. Browning, Sacramento, CA 2014/O1878O2 A1 7/2014 Mikochik et al. (US);US): Makoto N. Masuno,M. s Elk Grove, s 2015/02668432015,0203462 A1 9/20157/2015 CahanaBrowning et etal. al. CA (US); Ryan L. Smith, Sacramento, 2016/0002190 A1 1/2016 Browning et al. CA (US); John Bissell, II, Sacramento, 2016,01681 07 A1 6/2016 Masuno et al. CA (US) 2016/02O7897 A1 7, 2016 Wood et al. (73) Assignee: MICROMIDAS, INC., West FOREIGN PATENT DOCUMENTS Sacramento, CA (US) CN 101475544. A T 2009 (*) Notice: Subject to any disclaimer, the term of this N 1939: A 658 patent is extended or adjusted under 35 DE 635,783 C 9, 1936 U.S.C. 154(b) by 0 days. EP 291494 A2 11, 1988 EP 1049657 B1 3, 2003 (21) Appl. No.: 14/852,306 GB 1220851 A 1, 1971 GB 1448489 A 9, 1976 RU 2429234 C2 9, 2011 (22) Filed: Sep. -

Refinery Wastewater Management Using Multiple Angle Oil-Water Separators
REFINERY WASTEWATER MANAGEMENT USING MULTIPLE ANGLE OIL-WATER SEPARATORS Kirby S. Mohr, P.E. Mohr Separations Research, Inc. 1278 FM 407 Suite 109 Lewisville, TX 75077 Phone: 918-299-9290 Cell: 918-269-8710 John N. Veenstra, Ph.D., P.E., Oklahoma State University Dee Ann Sanders, Ph.D., P.E. Oklahoma State University A paper presented at the International Petroleum Environment Conference in Albuquerque, New Mexico, 1998 ABSTRACT In this work, an overview of oil-water separation, as used in the petroleum refining industries, is presented along with case studies. Discussions include: impact of solids, legal aspects, and differing types of systems currently in use, along with their advantages and disadvantages. Performance information on separators is presented with an emphasis on new multiple angle coalescing plate technology for refinery wastewater management. Several studies are presented including a large (20,000 US GPM flow rate) system recently installed at a major US refinery. The separator was constructed by converting two existing API separators into four separators, and adding multiple angle coalescing plates to increase throughput and efficiency. A year of operating experience with this system indicates good performance and few problems. Other examples provide information on separators installed in the United States and other countries. Keywords: Oil-water separator, multiple angle, coalescence, refinery, wastewater management, petroleum, coalescing plate technology BACKGROUND AND INTRODUCTION Oil has been refined for various uses for at least 1000 years. An Arab handbook written by Al-Razi, in approximately 865 A.D., describes distillation of “naft” (naphtha) for use in lamps and thus the beginning of oil refining (Forbes). -

Distillation Theory
Chapter 2 Distillation Theory by Ivar J. Halvorsen and Sigurd Skogestad Norwegian University of Science and Technology Department of Chemical Engineering 7491 Trondheim, Norway This is a revised version of an article published in the Encyclopedia of Separation Science by Aca- demic Press Ltd. (2000). The article gives some of the basics of distillation theory and its purpose is to provide basic understanding and some tools for simple hand calculations of distillation col- umns. The methods presented here can be used to obtain simple estimates and to check more rigorous computations. NTNU Dr. ing. Thesis 2001:43 Ivar J. Halvorsen 28 2.1 Introduction Distillation is a very old separation technology for separating liquid mixtures that can be traced back to the chemists in Alexandria in the first century A.D. Today distillation is the most important industrial separation technology. It is particu- larly well suited for high purity separations since any degree of separation can be obtained with a fixed energy consumption by increasing the number of equilib- rium stages. To describe the degree of separation between two components in a column or in a column section, we introduce the separation factor: ()⁄ xL xH S = ------------------------T (2.1) ()x ⁄ x L H B where x denotes mole fraction of a component, subscript L denotes light compo- nent, H heavy component, T denotes the top of the section, and B the bottom. It is relatively straightforward to derive models of distillation columns based on almost any degree of detail, and also to use such models to simulate the behaviour on a computer. -

By a 965% ATT'ys
July 19, 1960 A. WATZL ETAL 2,945,788 PROCESS FOR THE PURIFICATION OF DIMETHYLTEREPHTHALATE Filed Nov. 19, l956 AZEOTROPE DISTILLATE CONDENSER CONDENSED WACUUM DISTILLATE DRY DISTILLATION MXTURE N2 COLUMN MPURE - SOD WACUUMFILTER ETHYLENE DMETHYL DMETHYL GLYCOL TEREPHTHALATE TEREPHTHALATE ETHYLENE GLYCOL INVENTORS: ANTON WATZL by aERHARD 965% SIGGEL ATT'YS 2,945,788 Patented July 19, 1960 2 phthalate is immediately suitable for reesterification or Subsequent polycondensation to polyethylene terephthal 2,945,788 ate. There are gained polycondensates of high degree of PROCESS FOR THE PURIFICATION OF viscosity with K values from 50 to 57. DMETHYLTEREPHTHALATE The best mode contemplated for practicing the inven Anton Watz, Kleinwallstadt (Ufr), and Erhard Siggel, tion involves the use of ethylene glycol as the aliphatic Laudenbach (Main), Germany, assignors to Vereinigte glycol. The following is a specific illustration thereof. Glanzstoff-Fabriken A.G., Wuppertal-Elberfeld, Ger Example many Thirty grams of crude dimethylterephthalate are mixed Filed Nov.19, 1956, Ser. No. 622,778 in a flask with 270 grams of ethylene glycol and azeo tropically distilled with the introduction of dry nitrogen 4 Claims. (C. 202-42) in a column of 30 cm. at about 44 Torr (1 Torr equals 1 mm. Hg). The azeotrope goes over at about 120° C. i This invention, in general, relates to production of into a cooled condenser. The dimethylterephthalate dimethylterephthalate and more particularly to the puri 5 separated from the glycol by vacuum filtering can be fication thereof. used immediately for reesterification or for polyconden The purification of dimethylterephthalate can be car sation. This process is illustrated in the flow sheet of ried out either by distillation or by recrystallization from the accompanying drawing.