Geochemistry of a Volcanic Hydrothermal System at Mount Spurr, Alaska
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 Paleobotanical Proxies for Early Eocene Climates and Ecosystems in Northern North 2 America from Mid to High Latitudes 3 4 Christopher K
https://doi.org/10.5194/cp-2020-32 Preprint. Discussion started: 24 March 2020 c Author(s) 2020. CC BY 4.0 License. 1 Paleobotanical proxies for early Eocene climates and ecosystems in northern North 2 America from mid to high latitudes 3 4 Christopher K. West1, David R. Greenwood2, Tammo Reichgelt3, Alexander J. Lowe4, Janelle M. 5 Vachon2, and James F. Basinger1. 6 1 Dept. of Geological Sciences, University of Saskatchewan, 114 Science Place, Saskatoon, 7 Saskatchewan, S7N 5E2, Canada. 8 2 Dept. of Biology, Brandon University, 270-18th Street, Brandon, Manitoba R7A 6A9, Canada. 9 3 Department of Geosciences, University of Connecticut, Beach Hall, 354 Mansfield Rd #207, 10 Storrs, CT 06269, U.S.A. 11 4 Dept. of Biology, University of Washington, Seattle, WA 98195-1800, U.S.A. 12 13 Correspondence to: C.K West ([email protected]) 14 15 Abstract. Early Eocene climates were globally warm, with ice-free conditions at both poles. Early 16 Eocene polar landmasses supported extensive forest ecosystems of a primarily temperate biota, 17 but also with abundant thermophilic elements such as crocodilians, and mesothermic taxodioid 18 conifers and angiosperms. The globally warm early Eocene was punctuated by geologically brief 19 hyperthermals such as the Paleocene-Eocene Thermal Maximum (PETM), culminating in the 20 Early Eocene Climatic Optimum (EECO), during which the range of thermophilic plants such as 21 palms extended into the Arctic. Climate models have struggled to reproduce early Eocene Arctic 22 warm winters and high precipitation, with models invoking a variety of mechanisms, from 23 atmospheric CO2 levels that are unsupported by proxy evidence, to the role of an enhanced 24 hydrological cycle to reproduce winters that experienced no direct solar energy input yet remained 25 wet and above freezing. -

Alaska Division of Geological & Geophysical Surveys Geologic
Cretaceous to Tertiary magmatism and associated mineralization in the Lime Hills C-1 Quadrangle, Western Alaska Range Karri R. Sicard 1, Evan Twelker1, Larry K. Freeman1, Alicja Wypych1, Jeff A. Benowitz2 , and M. Andy Kass3 1Alaska Division of Geological & Geophysical Surveys, Fairbanks, Alaska 2Geophysical Institute, Department of Geology & Geophysics, University of Alaska Fairbanks 3Crustal Geophysics and Geochemistry Science Center, US Geological Survey, Denver, CO Alaska Miners Association Annual Convention 1 November 5, 2014 – Anchorage, Alaska Outline ► Location and geology ► Mineralization highlights ► New 40Ar/39Ar geochronology . With relevant geochemistry ► 3D voxel model of Copper Joe resistivity ► Relationship to Revelation Mountains uplift ► Acknowledgments 2 Photo by T.C. Wright Cretaceous-Tertiary Porphyry Trend ~70 Ma Select Northern Cordillera ~90 Ma porphyry copper deposits ~6 Ma 3 http://www.kiskametals.com Terrane Map YUKON-TANANA TERRANE McGrath WRANGELLIA Project Area Anchorage 4 Modified from Colpron and Nelson, 2011 Denali-Farewell Fault System Jurassic-Cretaceous Revelation flysch Mountains Lime Hills C-1 Cretaceous and Tertiary Quad plutonic rocks Tordrillo Mountains Tertiary volcanics 5 Map modified from Wilson and others, 1998 Airborne Geophysical Surveys Farewell (Burns and others, 2014) Middle Styx (Burns and others, 2013) East Styx More positive magnetic values (Released November 2014) Styx Lime Hills (Burns and More negative others 2008) 6 magnetic values C-1 Quad nT Anomalous Gold Occurrences Circles -

Geologic Maps of the Eastern Alaska Range, Alaska, (44 Quadrangles, 1:63360 Scale)
Report of Investigations 2015-6 GEOLOGIC MAPS OF THE EASTERN ALASKA RANGE, ALASKA, (44 quadrangles, 1:63,360 scale) descriptions and interpretations of map units by Warren J. Nokleberg, John N. Aleinikoff, Gerard C. Bond, Oscar J. Ferrians, Jr., Paige L. Herzon, Ian M. Lange, Ronny T. Miyaoka, Donald H. Richter, Carl E. Schwab, Steven R. Silva, Thomas E. Smith, and Richard E. Zehner Southeastern Tanana Basin Southern Yukon–Tanana Upland and Terrane Delta River Granite Jarvis Mountain Aurora Peak Creek Terrane Hines Creek Fault Black Rapids Glacier Jarvis Creek Glacier Subterrane - Southern Yukon–Tanana Terrane Windy Terrane Denali Denali Fault Fault East Susitna Canwell Batholith Glacier Maclaren Glacier McCallum Creek- Metamorhic Belt Meteor Peak Slate Creek Thrust Broxson Gulch Fault Thrust Rainbow Mountain Slana River Subterrane, Wrangellia Terrane Phelan Delta Creek River Highway Slana River Subterrane, Wrangellia Terrane Published by STATE OF ALASKA DEPARTMENT OF NATURAL RESOURCES DIVISION OF GEOLOGICAL & GEOPHYSICAL SURVEYS 2015 GEOLOGIC MAPS OF THE EASTERN ALASKA RANGE, ALASKA, (44 quadrangles, 1:63,360 scale) descriptions and interpretations of map units Warren J. Nokleberg, John N. Aleinikoff, Gerard C. Bond, Oscar J. Ferrians, Jr., Paige L. Herzon, Ian M. Lange, Ronny T. Miyaoka, Donald H. Richter, Carl E. Schwab, Steven R. Silva, Thomas E. Smith, and Richard E. Zehner COVER: View toward the north across the eastern Alaska Range and into the southern Yukon–Tanana Upland highlighting geologic, structural, and geomorphic features. View is across the central Mount Hayes Quadrangle and is centered on the Delta River, Richardson Highway, and Trans-Alaska Pipeline System (TAPS). Major geologic features, from south to north, are: (1) the Slana River Subterrane, Wrangellia Terrane; (2) the Maclaren Terrane containing the Maclaren Glacier Metamorphic Belt to the south and the East Susitna Batholith to the north; (3) the Windy Terrane; (4) the Aurora Peak Terrane; and (5) the Jarvis Creek Glacier Subterrane of the Yukon–Tanana Terrane. -

Fossil Mosses: What Do They Tell Us About Moss Evolution?
Bry. Div. Evo. 043 (1): 072–097 ISSN 2381-9677 (print edition) DIVERSITY & https://www.mapress.com/j/bde BRYOPHYTEEVOLUTION Copyright © 2021 Magnolia Press Article ISSN 2381-9685 (online edition) https://doi.org/10.11646/bde.43.1.7 Fossil mosses: What do they tell us about moss evolution? MicHAEL S. IGNATOV1,2 & ELENA V. MASLOVA3 1 Tsitsin Main Botanical Garden of the Russian Academy of Sciences, Moscow, Russia 2 Faculty of Biology, Lomonosov Moscow State University, Moscow, Russia 3 Belgorod State University, Pobedy Square, 85, Belgorod, 308015 Russia �[email protected], https://orcid.org/0000-0003-1520-042X * author for correspondence: �[email protected], https://orcid.org/0000-0001-6096-6315 Abstract The moss fossil records from the Paleozoic age to the Eocene epoch are reviewed and their putative relationships to extant moss groups discussed. The incomplete preservation and lack of key characters that could define the position of an ancient moss in modern classification remain the problem. Carboniferous records are still impossible to refer to any of the modern moss taxa. Numerous Permian protosphagnalean mosses possess traits that are absent in any extant group and they are therefore treated here as an extinct lineage, whose descendants, if any remain, cannot be recognized among contemporary taxa. Non-protosphagnalean Permian mosses were also fairly diverse, representing morphotypes comparable with Dicranidae and acrocarpous Bryidae, although unequivocal representatives of these subclasses are known only since Cretaceous and Jurassic. Even though Sphagnales is one of two oldest lineages separated from the main trunk of moss phylogenetic tree, it appears in fossil state regularly only since Late Cretaceous, ca. -
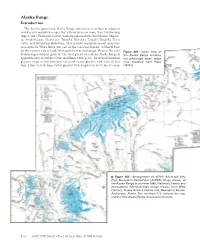
Alaska Range
Alaska Range Introduction The heavily glacierized Alaska Range consists of a number of adjacent and discrete mountain ranges that extend in an arc more than 750 km long (figs. 1, 381). From east to west, named ranges include the Nutzotin, Mentas- ta, Amphitheater, Clearwater, Tokosha, Kichatna, Teocalli, Tordrillo, Terra Cotta, and Revelation Mountains. This arcuate mountain massif spans the area from the White River, just east of the Canadian Border, to Merrill Pass on the western side of Cook Inlet southwest of Anchorage. Many of the indi- Figure 381.—Index map of vidual ranges support glaciers. The total glacier area of the Alaska Range is the Alaska Range showing 2 approximately 13,900 km (Post and Meier, 1980, p. 45). Its several thousand the glacierized areas. Index glaciers range in size from tiny unnamed cirque glaciers with areas of less map modified from Field than 1 km2 to very large valley glaciers with lengths up to 76 km (Denton (1975a). Figure 382.—Enlargement of NOAA Advanced Very High Resolution Radiometer (AVHRR) image mosaic of the Alaska Range in summer 1995. National Oceanic and Atmospheric Administration image mosaic from Mike Fleming, Alaska Science Center, U.S. Geological Survey, Anchorage, Alaska. The numbers 1–5 indicate the seg- ments of the Alaska Range discussed in the text. K406 SATELLITE IMAGE ATLAS OF GLACIERS OF THE WORLD and Field, 1975a, p. 575) and areas of greater than 500 km2. Alaska Range glaciers extend in elevation from above 6,000 m, near the summit of Mount McKinley, to slightly more than 100 m above sea level at Capps and Triumvi- rate Glaciers in the southwestern part of the range. -

USGS Professional Paper 1739-A
Studies by the U.S. Geological Survey in Alaska, 2006 U.S. Geological Survey Professional Paper 1739–A Blue Mountain and The Gas Rocks: Rear-Arc Dome Clusters on the Alaska Peninsula By Wes Hildreth, Judy Fierstein, and Andrew T. Calvert Abstract pal nearby town) and 15 to 20 km behind (northwest of) the volcanic-front chain, which is locally defined by Kejulik and Behind the single-file chain of stratovolcanoes on the Peulik stratovolcanoes (fig. 1). The Gas Rocks form a knobby Alaska Peninsula, independent rear-arc vents for mafic mag- peninsula at the south shore of Becharof Lake, and Blue mas are uncommon, and for silicic magmas rarer still. We Mountain is a group of rounded hills a few kilometers west of report here the characteristics, compositions, and ages of two Upper Ugashik Lake (fig. 2). Both dome clusters rise abruptly andesite-dacite dome clusters and of several nearby basaltic above a nearly flat (virtually treeless and roadless) plain of units, all near Becharof Lake and 15 to 20 km behind the late Pleistocene glacial deposits (Detterman and others, 1987a, volcanic front. Blue Mountain consists of 13 domes (58–68 b), consisting largely of till and outwash, supplemented by the bog and lacustrine deposits of hundreds of ponds and by weight percent SiO2) and The Gas Rocks of three domes (62–64.5 weight percent SiO ) and a mafic cone (52 weight beach and terrace deposits along the lakeshores. The enor- 2 mous moraine-dammed lakes (fig. 2) are generally shallower percent SiO2). All 16 domes are amphibole-biotite-plagio- clase felsite, and nearly all are phenocryst rich and quartz than 5 m, and their surfaces are barely 10 m above sea level. -

The Geology, Paleontology and Paleoecology of the Cerro Fortaleza Formation
The Geology, Paleontology and Paleoecology of the Cerro Fortaleza Formation, Patagonia (Argentina) A Thesis Submitted to the Faculty of Drexel University by Victoria Margaret Egerton in partial fulfillment of the requirements for the degree of Doctor of Philosophy November 2011 © Copyright 2011 Victoria M. Egerton. All Rights Reserved. ii Dedications To my mother and father iii Acknowledgments The knowledge, guidance and commitment of a great number of people have led to my success while at Drexel University. I would first like to thank Drexel University and the College of Arts and Sciences for providing world-class facilities while I pursued my PhD. I would also like to thank the Department of Biology for its support and dedication. I would like to thank my advisor, Dr. Kenneth Lacovara, for his guidance and patience. Additionally, I would like to thank him for including me in his pursuit of knowledge of Argentine dinosaurs and their environments. I am also indebted to my committee members, Dr. Gail Hearn, Dr. Jake Russell, Dr. Mike O‘Connor, Dr. Matthew Lamanna, Dr. Christopher Williams and Professor Hermann Pfefferkorn for their valuable comments and time. The support of Argentine scientists has been essential for allowing me to pursue my research. I am thankful that I had the opportunity to work with such kind and knowledgeable people. I would like to thank Dr. Fernando Novas (Museo Argentino de Ciencias Naturales) for helping me obtain specimens that allowed this research to happen. I would also like to thank Dr. Viviana Barreda (Museo Argentino de Ciencias Naturales) for her allowing me use of her lab space while I was visiting Museo Argentino de Ciencias Naturales. -

Terrestrial Mollusks of Attu, Aleutian Islands, Alaska BARRY ROTH’ and DAVID R
ARCTK: VOL. 34, NO. 1 (MARCH 1981), P. 43-47 Terrestrial Mollusks of Attu, Aleutian Islands, Alaska BARRY ROTH’ and DAVID R. LINDBERG’ ABSTRACT. Seven species of land mollusk (2 slugs, 5 snails) were collected on Attu in July 1979. Three are circumboreal species, two are amphi-arctic (Palearctic and Nearctic but not circumboreal), and two are Nearctic. Barring chance survival of mollusks in local refugia, the fauna was assembled overwater since deglaciation, perhaps within the last 10 OOO years. Mollusk faunas from Kamchatka to southeastern Alaska all have a Holarctic component. A Palearctic component present on Kamchatka and the Commander Islands is absent from the Aleutians, which have a Nearctic component that diminishes westward. This pattern is similar to that of other soil-dwelling invertebrate groups. RESUM& Sept espbces de mollusques terrestres (2 limaces et 5 escargots) furent prklevkes sur I’ile d’Attu en juillet 1979. Trois sont des espbces circomborkales, deux amphi-arctiques (Palkarctiques et Nkarctiques mais non circomborkales), et deux Nkarctiques. Si I’on excepte la survivance de mollusques due auhasard dans des refuges locaux, cette faune s’est retrouvke de part et d’autre des eauxdepuis la dkglaciation, peut-&re depuis les derniers 10 OOO ans. Les faunes de mollusques de la pkninsule de Kamchatkajusqu’au sud-est de 1’Alaska on toutes une composante Holarctique. Une composante Palkarctique prksente sur leKamchatka et les iles Commandeur ne se retrouve pas aux Alkoutiennes, oil la composante Nkarctique diminue vers I’ouest. Ce patron est similaire il celui de d’autres groupes d’invertkbrks terrestres . Traduit par Jean-Guy Brossard, Laboratoire d’ArchCologie de I’Universitk du Qukbec il Montrkal. -

A New Satellite-Derived Glacier Inventory for Western Alaska
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2011 A new satellite-derived glacier inventory for western Alaska Le Bris, R ; Paul, F ; Frey, H ; Bolch, T Abstract: Glacier inventories provide the baseline data to perform climate-change impact assessment on a regional scale in a consistent and spatially representative manner. In particular, a more accurate calculation of the current and future contribution to global sea-level rise from heavily glacierized re- gions such as Alaska is much needed. We present a new glacier inventory for a large part of western Alaska (including Kenai Peninsula and the Tordrillo, Chigmit and Chugach mountains), derived from nine Landsat Thematic Mapper scenes acquired between 2005 and 2009 using well-established automated glacier-mapping techniques (band ratio). Because many glaciers are covered by optically thick debris or volcanic ash and partly calve into water, outlines were manually edited in these wrongly classified regions during post-processing. In total we mapped 8830 glaciers (>0.02 km2) with a total area of 16 250 km2. Large parts of the area (47%) are covered by a few (31) large (>100 km2) glaciers, while glaciers less than 1 km2 constitute only 7.5% of the total area but 86% of the total number. We found a strong dependence of mean glacier elevation on distance from the ocean and only a weak one on aspect. Glacier area changes were calculated for a subset of 347 selected glaciers by comparison with the Digital Line Graph outlines from the US Geological Survey. -

City of Wasilla Hazard Mitigation Plan (Phase I – Natural Hazards)
City of Wasilla Hazard Mitigation Plan (Phase I – Natural Hazards) 2018 Update by: Wasilla Planning Commission Acknowledgements Wasilla City Council Bert Cottle, Mayor Glenda Ledford Tim Burney Stu Graham Mike Dryden Gretchen O’Barr James E. Harvey Wasilla Planning Commission Eric Bushnell Darrell L. Breese Jessica Dean Simon Brown Brian L. Mayer City Staff Tina Crawford, City Planner City of Wasilla Planning Office 290 E. Herning Avenue Wasilla, AK 99654 Phone: (907) 373-9020 Fax: (907) 373-9021 E-Mail: [email protected] Consultants LeMay Engineering & Consulting, Inc. Jennifer LeMay, PE, PMP John Farr, EIT 4272 Chelsea Way Anchorage, Alaska 99504 Phone: (907) 350-6061 Email: [email protected]; [email protected] Technical Assistance Brent Nichols, CFM, SHMO, Alaska State DHS&EM The preparation of this plan was financed by funds from a grant from the Alaska Division of Homeland Security and Emergency Management and the Federal Emergency Management Agency. ii City of Wasilla 2018 Hazard Mitigation Plan Acronyms °F Degrees Fahrenheit ADEC Alaska Department of Environmental Conservation AEIC Alaska Earthquake Information Center AFS Alaska Fire Service AHS Alaska Hydrologic Survey APA Approved Pending Adoption AS Alaska State Statute AKST Alaska Standard Time BCA Benefit-Cost Analysis BCR Benefit-Cost Ratio BLM Bureau of Land Management CFR Code of Federal Regulations DCCED Department of Commerce, Community, and Economic Development (State of Alaska) DHS&EM Department of Homeland Security and Emergency Management -

United States Department of the Interior Geological
UNITED STATES DEPARTMENT OF THE INTERIOR GEOLOGICAL SURVEY AGE AND TECTONIC SIGNIFICANCE OF VOLCANIC ROCKS ON ST. MATTHEW ISLAND, BERING SEA, ALASKA BY William W. Patton, Jr., Marvin A. Lanphere, Thomas P. Miller, and Richard A. Scott Menlo Park, Calif., Anchorage, Alaska, Denver, Colo. Open-file report 75-150 1975 This report is preliminary and has not been edited or reviewed for conformity with Geological Survey standards AGE AND TECTONIC SIGNIFICANCE OF VOLCANIC ROCKS ON ST. MATTHEW ISLAND, BERING SEA, ALASKA By WILLIAM W. PATTON, JR., MARVIN A. LANPHERE, THOMAS P. MILLER, and RICHARD A. SCOTT Menlo Park, Calif., Anchorage, Alaska, Denver, Colo. Abstract.-- 'Reconnaissance investigations of the heretofore little known volcanic assemblage on St. Matthew Island provide significant information on the tectonic history of the Bering Sea shelf. St. Matfhew Island is made up of approximately 500 m of subaerial calc-alkaline volcanic rocks ranging in composition from high-alumina basalt to rhyolite. Four K-Ar analyses of samples from this volcanic sequence give Late Cretaceous ages of 65-77 m.y., and intercalated carbonaceous tuff layers yield Cretaceous pollen assemblages. Along the northeast coast of St. Matthew Island the volcanic rocks are intruded by granodiorite that gives an early Tertiary K-Ar age of 61 m.y. Correlations with on-land geology in northeast Siberia and marine geophysical data from the western Bering Sea strongly suggest that St. Matthew Island represents a southeastward extension of the Okhotsk- Chukotsk belt, a Late Cretaceous and early Tertiary volcanic arc that borders the Pacific margin of Siberia for 3,000 km. -

Retallack 2021 Coal Balls
Palaeogeography, Palaeoclimatology, Palaeoecology 564 (2021) 110185 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Modern analogs reveal the origin of Carboniferous coal balls Gregory Retallack * Department of Earth Science, University of Oregon, Eugene, Oregon 97403-1272, USA ARTICLE INFO ABSTRACT Keywords: Coal balls are calcareous peats with cellular permineralization invaluable for understanding the anatomy of Coal ball Pennsylvanian and Permian fossil plants. Two distinct kinds of coal balls are here recognized in both Holocene Histosol and Pennsylvanian calcareous Histosols. Respirogenic calcite coal balls have arrays of calcite δ18O and δ13C like Carbon isotopes those of desert soil calcic horizons reflecting isotopic composition of CO2 gas from an aerobic microbiome. Permineralization Methanogenic calcite coal balls in contrast have invariant δ18O for a range of δ13C, and formed with anaerobic microbiomes in soil solutions with bicarbonate formed by methane oxidation and sugar fermentation. Respiro genic coal balls are described from Holocene peats in Eight Mile Creek South Australia, and noted from Carboniferous coals near Penistone, Yorkshire. Methanogenic coal balls are described from Carboniferous coals at Berryville (Illinois) and Steubenville (Ohio), Paleocene lignites of Sutton (Alaska), Eocene lignites of Axel Heiberg Island (Nunavut), Pleistocene peats of Konya (Turkey), and Holocene peats of Gramigne di Bando (Italy). Soils and paleosols with coal balls are neither common nor extinct, but were formed by two distinct soil microbiomes. 1. Introduction and Royer, 2019). Although best known from Euramerican coal mea sures of Pennsylvanian age (Greb et al., 1999; Raymond et al., 2012, Coal balls were best defined by Seward (1895, p.