New Mineral Names*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Alamosite Pbsio3 C 2001 Mineral Data Publishing, Version 1.2 ° Crystal Data: Monoclinic
Alamosite PbSiO3 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Monoclinic. Point Group: 2=m: Crystals ¯brous [010]; as radiating aggregates and balls, to 7.5 cm. k Physical Properties: Cleavage: Perfect on 010 . Hardness = 4.5 D(meas.) = 6.488(3) D(calc.) = [6.30] f g Optical Properties: Transparent to translucent. Color: Colorless to white, cream, or light gray. Luster: Adamantine. Optical Class: Biaxial ({). Orientation: Y = b. Dispersion: r < v; strong, weak inclined. ® = 1.945{1.947 ¯ = 1.955{1.961 ° = 1.959{1.968 2V(meas.) = 65± Cell Data: Space Group: P 2=n: a = 12.247 b = 7.059 c = 11.236 ¯ = 113:12± Z = 12 X-ray Powder Pattern: Tsumeb, Namibia. 3.34 (100), 3.56 (95), 3.53 (75), 2.300 (75), 3.23 (70), 2.987 (70), 3.25 (60) Chemistry: (1) (2) (3) SiO2 21.11 20.01 21.21 Al2O3 0.09 FeO 0.09 0.18 MnO 0.02 PbO 78.13 78.95 78.79 CaO trace 0.09 insol: 0.61 Total 99.94 99.34 100.00 (1) Alamos, Mexico. (2) Tsumeb, Namibia; by electron microprobe. (3) PbSiO3: Occurrence: As a rare secondary mineral in the oxidized zone of lead-bearing base metal deposits. Association: Wulfenite, leadhillite, cerussite (Alamos, Mexico); leadhillite, anglesite, melanotekite, °eischerite, kegelite, hematite (Tsumeb, Namibia); diaboleite, phosgenite, cerussite, wulfenite, willemite (Tiger, Arizona, USA); melanotekite, shattuckite, wickenburgite (Rawhide mine, Arizona, USA). Distribution: From Mexico, in Sonora, at Alamos, and the San Pascual mine, near Zimap¶an, Hidalgo. In the USA, from Arizona, in the Mammoth-St. -

IMA–CNMNC Approved Mineral Symbols
Mineralogical Magazine (2021), 85, 291–320 doi:10.1180/mgm.2021.43 Article IMA–CNMNC approved mineral symbols Laurence N. Warr* Institute of Geography and Geology, University of Greifswald, 17487 Greifswald, Germany Abstract Several text symbol lists for common rock-forming minerals have been published over the last 40 years, but no internationally agreed standard has yet been established. This contribution presents the first International Mineralogical Association (IMA) Commission on New Minerals, Nomenclature and Classification (CNMNC) approved collection of 5744 mineral name abbreviations by combining four methods of nomenclature based on the Kretz symbol approach. The collection incorporates 991 previously defined abbreviations for mineral groups and species and presents a further 4753 new symbols that cover all currently listed IMA minerals. Adopting IMA– CNMNC approved symbols is considered a necessary step in standardising abbreviations by employing a system compatible with that used for symbolising the chemical elements. Keywords: nomenclature, mineral names, symbols, abbreviations, groups, species, elements, IMA, CNMNC (Received 28 November 2020; accepted 14 May 2021; Accepted Manuscript published online: 18 May 2021; Associate Editor: Anthony R Kampf) Introduction used collection proposed by Whitney and Evans (2010). Despite the availability of recommended abbreviations for the commonly Using text symbols for abbreviating the scientific names of the studied mineral species, to date < 18% of mineral names recog- chemical elements -

New Mineral Names*,†
American Mineralogist, Volume 102, pages 1961–1968, 2017 New Mineral Names*,† DMITRIY I. BELAKOVSKIY1, FERNANDO CÁMARA2, OLIVIER C. GAGNE3, AND YULIA UVAROVA4 1Fersman Mineralogical Museum, Russian Academy of Sciences, Leninskiy Prospekt 18 korp. 2, Moscow 119071, Russia 2Dipartimento di Scienze della Terra “Ardito Desio”, Universitá di degli Studi di Milano, Via Mangiagalli, 34, 20133 Milano, Italy 3Department of Geological Sciences, University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada 4CSIRO Mineral Resources, CSIRO, ARRC, 26 Dick Perry Avenue, Kensington, Western Australia 6151 Australia IN THIS ISSUE This New Mineral Names has entries for 14 new minerals, including bohseite, dachiardite-K, ilyukhinite, jahnsite-(CaFeMg), ježekite, karpenkoite, khesinite, mesaite, norilskite, plavnoite, raygrantite, shumwayite, steinmetzite, and tinnunculite. BOHSEITE* along with the average of 10 electron probe WDS analysis (in bold) on the crystal used for the collection of the XRD data are: SiO 58.83 E. Szełęg, B. Zuzens, F.C. Hawthorne, A. Pieczka, A. Szuszkiewicz, 2 (58.04–59.47) / 57.41 (54.69–60.02), Al O 3.51 (1.87–6.49) / 3.51 K. Turniak, K. Nejbert, S.S. Ilnicki, H. Friis, E. Makovicky, 2 3 (2.91–4.17), CaO 24.61 (24.45–24.96) / 23.75 (23.53–23.91), Na O 0.07 M.T. Weller and M.-H. Lemée-Cailleau (2017) Bohseite, ideally 2 (0.01–0.13) / 0.18 (0.16–0.19), F 0.45 (0.20–0.69) / 0.55 (0.39–0.75), Ca Be Si O (OH) , from the Piława Górna quarry, the Góry Sowie 2 4 4 9 24 4 BeO 9.31 (7.75–10.24) / 9.07, H O 3.12 (2.68–3.42) / 3.05, O=F Block, SW Poland. -
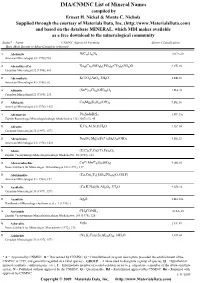
IMA/CNMNC List of Mineral Names
IMA/CNMNC List of Mineral Name s compiled by Ernest H. Nickel & Monte C. Nichols Supplied through the courtesy of Materials Data, Inc. (http://www.MaterialsData.com) and based on the database MINERAL, which MDI makes available as a free download to the mineralogical community Status* Name CNMNC Approved Formula Strunz Classification Best, Most Recent or Most Complete reference. A Abelsonite NiC£¡H£¢N¤ 10.CA.20 American Mineralogist 63 (1978) 930 A Abenakiite-(Ce) Na¢¦Ce¦(SiO£)¦(PO¤)¦(CO£)¦(SO¢)O 9.CK.10 Canadian Mineralogist 32 (1994), 843 G Abernathyite K(UO¢)AsO¤•3H¢O 8.EB.15 American Mineralogist 41 (1956), 82 A Abhurite (SnÀÈ)¢¡Cl¡¦(OH)¡¤O¦ 3.DA.30 Canadian Mineralogist 23 (1985), 233 D Abkhazite Ca¢Mg¥Si¨O¢¢(OH)¢ 9.DE.10 American Mineralogist 63 (1978), 1023 A Abramovite Pb¢SnInBiS§ 2.HF.25a Zapiski Rossiiskogo Mineralogicheskogo Obshchetstva 136 (2007) (5), 45 D Abrazite K,Ca,Al,Si,O,H¢O 9.GC.05 Canadian Mineralogist 35 (1997), 1571 D Abriachanite Na¢(Fe,Mg)£(FeÁÈ)¢Si¨O¢¢(OH)¢ 9.DE.25 American Mineralogist 63 (1978), 1023 D Absite (U,Ca,Y,Ce)(Ti,Fe)¢O¦ Zapiski Vsesoyuznogo Mineralogicheskogo Obshchestva 92 (1963), 113 A Abswurmbachite CuÀÈ(MnÁÈ)¦O¨(SiO¤) 9.AG.05 Neues Jahrbuch für Mineralogie, Abhandlungen 163 (1991), 117 D Abukumalite (Ca,Ce)¢Y£(SiO¤,PO¤)£(O,OH,F) American Mineralogist 51 (1966), 152 D Acadialite (Ca,K,Na)(Si,Al)£O¦•3H¢O 9.GD.10 Canadian Mineralogist 35 (1997), 1571 G Acanthite Ag¢S 2.BA.30a Handbook of Mineralogy (Anthony et al.), 1 (1990), 1 A Acetamide CH£CONH¢ 10.AA.20 Zapiski Vsesoyuznogo Mineralogicheskogo -

Full Text (224 K)
American Mineralogist, Volume 91, pages 216–224, 2006 New Mineral Names* ANDREW J. LOCOCK,1 PAULA C. PIILONEN,2,† T. SCOTT ERCIT,2,‡ AND RALPH ROWE2 1Department of Natural History, Royal Ontario Museum, 100 Queenʼs Park, Toronto, ON M5S 2C6, Canada 2Research Division, Canadian Museum of Nature, P.O. Box 3443, Station D, Ottawa, ON K1P 6P4, Canada ARAPOVITE* 5.34(23,100), 5.28(38,012), 3.37(100,120), 3.31(58,014), A.A. Agakhanov, Pautov, L.A., Uvarova, Y.A., Sokolova, E.V., 2.672(15,220), 2.640(64,024), 2.515(21,030), 2.493(15,124), Hawthorne, F.C., Karpenko, V.Yu., Dusmatov, Y.D., Semenov, 2.161(45,224), 2.016(29,232), 1.821(21,234), 1.689(16,240), 1.644(30,242), and 1.618(18,128). Arapovite is the U4+ ana- E.I. (2004) Arapovite, (U,Th)(Ca,Na)2(K1-x x) Si8O20·H2O— 4+ New mineral. New Data Mineral. M., 39, 14–19. logue of turkestanite and the U -Ca analogue of steacyite. The topology of the structure is identical to that of both turkestanite Y.A.Uvarova, E. Sokolova, F.C. Hawthorne, A.A. Agakha- and steacyite and consists of four-membered double rings of nov, L.A Pautov (2004) The crystal structure of arapovite, SiO4 tetrahedra in the form [Si8O20]. Eight coordinated A and B 4+ polyhedra share edges to form (001) sheets that are connected U (Ca,Na)2(K1-x x)[Si8O20], x ≈ 0.5, a new mineral species of the steacyite group from the Dara-i-Pioz moraine, Tien-Shan through [Si8O20] groups to form a framework. -

Download the Scanned
American Mineralogist, Volume 72, pages 1023-1028, 1987 NEW MINERAL NAMESX Fn-q.NxC. HawrsoRNE, JoHN Jarunon, KnNNnrrr W. Buon, Ennsr A. J. Bunxr, Jonr- D. GnrcnoDoN PHrr-r-rrs,ANonrw C. Rounnrs, Ronnnr A. Scsnur,nn Jltvrns E. Snrcr,nv Cameronite* corresponding to (on the basis of SO3 : l) (Mnoru,Mgoor- CaooorFeo*.f.r)"or77soo'6.39HrOor (on the basis of anhydrous A.C. Roberts, D.C. Harris, A.J. Criddle, W.W. Pinch (1986) O = 4) MnorroMgooorCaooorFeffir)rorrrSroorOo'6.42HrO.The Cameronite,a new copp€r-silvertelluride from the Good Hope ideal formula is (Mn,Mg)SO o'6H2Owith Mn > Mg and Z : 8. mine, Vulcan,Colorado. Can. Mineral., 24,379-384. DrA-rcA analysisshowed major peaksat 105 (HrO), 310 (HrO), Two closelymatching microprobe analysesof cameronite,ide- 987 (SO3),and 1000"C(SO.). Chvaleticeite(as described, magne- ally CurAgTe,o,give an averageof Cu 24.45,Ag 6.34,Te 69.I l, sian chvaleticeite)is the Mn-dominant member of the hexahy- sum 99.90 wt0/0,corresponding to Cu,oAg,orTe,oassuming 10 drite group. Te atoms. Single-crystalX-ray study indicates tetragonal sym- Material suitablefor single-crystalstudy was not found so that metry,a = 3a' : 12.695(2),c :7c' : 42.186(6)A, spacegroups unit-cell parameters were calculated by analogy with hexahy- P4r/mmc, P4rmc, or P4rc, D.",": 7.144 g/cm3 for the ideal drite. The mineral is monoclinic, space group C2/c wth a : formula with Z : 16. The strongest lines (26 given) are 10.05(2), b :7.24 (2),c : 24.3(1) A, B : 98.0(2f. -

New Mineral Names*
American Mineralogist, Volume 72, pages 1023-1028, 1987 NEW MINERAL NAMES* FRANK C. HAWTHORNE, JOHN JAMBOR, KENNETH W. BLADH, ERNST A. J. BURKE, JOEL D. GRICE, DON PHILLIPS, ANDREW C. ROBERTS, ROBERT A. SCHEDLER, JAMES E. SHIGLEY Cameronite* corresponding to (on the basis of S03 = 1) (Mno.567M80.4os- Cao.002Fet.J03):E0.977S04.6.39H20or (on the basis of anhydrous A.C. Roberts, D.C. Harris, A.J. Criddle, W.W. Pinch (1986) o = 4) MnO.570M80.407CaO.002Fet.J03):EO.982S1.00S04.6.42H20. The Cameronite, a new copper-silver telluride from the Good Hope ideal formula is (Mn,Mg)S04.6H20 with Mn > Mg and Z = 8. mine, Vulcan, Colorado. Can. Mineral., 24, 379-384. DTA-TGAanalysis showed major peaks at 105 (H20), 310 (H20), Two closely matching microprobe analyses of cameronite, ide- 987 (S03)' and 1000°C (S03). Chvaleticeite (as described, magne- ally Cu7AgTelO, give an average of Cu 24.45, Ag 6.34, Te 69.11, sian chvaleticeite) is the Mn-dominant member of the hexahy- sum 99.90 wtO/o,corresponding to CU7.lOAg1.09TelOassuming 10 drite group. Te atoms. Single-crystal X-ray study indicates tetragonal sym- Material suitable for single-crystal study was not found so that metry, a = 3a' = 12.695(2), c = 7c' = 42.186(6) A, space groups unit-cell parameters were. calculated by analogy with hexahy- P4/mmc, P4zmc, or P42c, Deale= 7.144 glcm3 for the ideal drite. The mineral is monoclinic, space group C2/c with a = formula with Z = 16. The strongest lines (26 given) are 10.05 (2), b = 7.24 (2), c = 24.3 (1) A, (3= 98.0(2)°.