Orchidaceae) Mark A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Typification of Infrageneric Taxa in Dendrobium(Orchidaceae)
Typification of infrageneric taxa in Dendrobium (Orchidaceae) André Schuiteman Royal Botanic Gardens, Kew, Richmond, Surrey, TW9 3AB, United Kingdom; e-mail: [email protected] Introduction Abstract The nomenclature of infrageneric taxa in Orchidaceae on occasion In order to stabilise the nomenclature borders on the chaotic. In the past, names were often proposed in a of infrageneric taxa in Dendrobium, type species are chosen for those highly informal fashion, with little concern for typification, priority, taxa for which this has not been precise circumscription, or even ranking. Such names were frequently done previously. In all, 35 names are applied to sets of species which had little in common beyond one or typified, including one genus name a few key characters. Those who used these names rarely cited their (Onychium), and assigned to sections authors, while the circumscription of the taxa often varied considerably of Dendrobium as recognised in Genera Orchidacearum. from one botanist to the next. It was not uncommon that earlier names were deliberately disregarded, to be replaced with new names that Keywords: Dendrobium, infrageneric taxa, nomenclature, taxonomy more or less covered the same groups. As a result, the nomenclature and systematics of many infrageneric taxa were, and sometimes still are, Muelleria 30(1): 3–7 (2012) extremely confused. In Dendrobium, one of the largest orchid genera with some 1500 species (Pridgeon et al., in prep.), well over 200 subgenera, sections and subsections have been proposed. Examples of all the problems just mentioned can be found in abundance in this gargantuan taxon. Fortunately, many of these problems can be solved rather easily by carefully typifying the taxa involved. -

The Diversity of Wild Orchids in the Southern Slope of Mount Merapi, Yogyakarta, Indonesia Eight Years After the 2010 Eruption
BIODIVERSITAS ISSN: 1412-033X Volume 21, Number 9, September 2020 E-ISSN: 2085-4722 Pages: 4457-4465 DOI: 10.13057/biodiv/d210964 The diversity of wild orchids in the southern slope of Mount Merapi, Yogyakarta, Indonesia eight years after the 2010 eruption FEBRI YUDA KURNIAWAN1,2,♥, FAUZANA PUTRI2,3, AHMAD SUYOKO2,3, HIMAWAN MASYHURI2,3, MAYA PURQI SULISTIANINGRUM2,3, ENDANG SEMIARTI3,♥♥ 1Postgraduate School, Universitas Gadjah Mada. Jl. Teknika Utara, Sleman 55281, Yogyakarta, Indonesia. Tel./fax. +62-274-544975, email: [email protected] 2Biology Orchid Study Club (BiOSC), Faculty of Biology, Universitas Gadjah Mada. Jl. Teknika Selatan, Sekip Utara, Sleman 55281, Yogyakarta, Indonesia 3Department of Tropical Biology, Faculty of Biology, Universitas Gadjah Mada. Jl. Teknika Selatan, Sekip Utara, Sleman 55281, Yogyakarta, Indonesia. Tel./fax.: +62-274-580839, email: [email protected] Manuscript received: 21 August 2020. Revision accepted: 31 August 2020. Abstract. Kurniawan FY, Putri F, Suyoko A, Masyhuri H, Sulistianingrum MP, Semiarti E. 2020. The diversity of wild orchids in the southern slope of Mount Merapi, Yogyakarta, Indonesia eight years after the 2010 eruption. Biodiversitas 21: 4457-4465. The ecosystem of the slopes of Mount Merapi is mountain tropical forest which is frequently affected by volcanic activities. The dynamics of the volcano affect the diversity and abundance of orchids in the ecosystem. Tritis is an area included in the Turgo Hill of the southern slope of Mount Merapi and is under the management of Mount Merapi National Park. The ecosystem in Tritis area classified as lower mountain forest and it has been affected by Mount Merapi eruption. This study aimed to do an inventory of orchid species in Tritis to know the diversity and abundance of orchids that exist in this area. -

Bulbophyllum Lamb, Spec
BLUMEA 38 (1994) 335-348 Notes on Bulbophylunae(Orchidaceae) from Borneo J.J. Vermeulen & A. Lamb Summary all and one ofthe Trias are described, originat- Nine new species of the genus Bulbophyllum genus are ing from Borneo. Notes on two more Bornean species of Bulbophyllum given. additional the informationon BorneanBulbophyllum species given This paper is to The Trias is in Vermeulen (1991). It adds nine new species to the checklist. genus Two other Bulbo- recorded for the first time from Borneo, with one new species. have been reduced into phyllum species are mentionedwhich appear to incorrectly synonymy in Vermeulen (1991). 1. Bulbophyllum habrotinum J. J. Vermeulen & A. Lamb, spec. nov. (sect. Cirrhopetalum) — Fig. 1 adaxialiter Bulbophyllum habrotinumJ.J. Vermeulen & A. Lamb, a B. makoyano in labello verru- — Leiden cult. (De 913245 (L). coso differt. Typus: Vogel) 1-2.5 0.9-2.8 Rhizome creeping, 2.5-3 mm diam.Pseudobulbs ovoid, cm apart, Petiole 5-10 7- by 0.6-1.1 cm, somewhat flattened or not. mm. Leaf blade elliptic, index obtuse. 4.5-9 20 by 2-3 cm, 3.5-10, tip Inflorescence usually single, cm, 8 Rhachis 6-9-flowered. Peduncle 4.3-8.7 cm; bracts c. 4, the longest c. mm. Floral bracts 4-5 Pedicel and nodding, 0.2-0.3 cm. ovate, mm, tip acute. ovary Flowers in all the little 6-7 mm. pendulous, a whorl, open at same time, opening. 3.5-4 index often Median sepal elliptic, 4.2-4.5 by mm, 1.1-1.3, tip acuminate, rather thin; surface Lateral with a terminal hair; margins long ciliate; glabrous. -

Dendrobium Kingianum Bidwill Ex Lindl
Volume 24: 203–232 ELOPEA Publication date: 19 May 2021 T dx.doi.org/10.7751/telopea14806 Journal of Plant Systematics plantnet.rbgsyd.nsw.gov.au/Telopea • escholarship.usyd.edu.au/journals/index.php/TEL • ISSN 0312-9764 (Print) • ISSN 2200-4025 (Online) A review of Dendrobium kingianum Bidwill ex Lindl. (Orchidaceae) with morphological and molecular- phylogenetic analyses Peter B. Adams1,2, Sheryl D. Lawson2, and Matthew A.M. Renner 3 1The University of Melbourne, School of BioSciences, Parkville 3010, Victoria 2National Herbarium of Victoria, Royal Botanic Gardens Victoria, Birdwood Ave., Melbourne 3004, Victoria 3National Herbarium of New South Wales, Royal Botanic Gardens and Domain Trust, Sydney 2000, New South Wales Author for correspondence: [email protected] Abstract Populations of Dendrobium kingianum Bidwill ex Lindl. from near Newcastle, New South Wales to southern and central west Queensland and encompassing all regions of the distribution were studied using field observations, morphometric analysis and nrITS sequences. A total of 281 individuals were used to construct regional descriptions of D. kingianum and 139 individuals were measured for 19 morphological characters, and similarities and differences among specimens summarised using multivariate statistical methods. Patterns of morphological variation within D. kingianum are consistent with a single variable species that expresses clinal variation, with short-growing plants in the south and taller plants in the northern part of the distribution. The nrITS gene tree suggests two subgroups within D. kingianum subsp. kingianum, one comprising northern, the other southern individuals, which may overlap in the vicinity of Dorrigo, New South Wales. The disjunct D. kingianum subsp. carnarvonense Peter B. -

Australia Lacks Stem Succulents but Is It Depauperate in Plants With
Available online at www.sciencedirect.com ScienceDirect Australia lacks stem succulents but is it depauperate in plants with crassulacean acid metabolism (CAM)? 1,2 3 3 Joseph AM Holtum , Lillian P Hancock , Erika J Edwards , 4 5 6 Michael D Crisp , Darren M Crayn , Rowan Sage and 2 Klaus Winter In the flora of Australia, the driest vegetated continent, [1,2,3]. Crassulacean acid metabolism (CAM), a water- crassulacean acid metabolism (CAM), the most water-use use efficient form of photosynthesis typically associated efficient form of photosynthesis, is documented in only 0.6% of with leaf and stem succulence, also appears poorly repre- native species. Most are epiphytes and only seven terrestrial. sented in Australia. If 6% of vascular plants worldwide However, much of Australia is unsurveyed, and carbon isotope exhibit CAM [4], Australia should host 1300 CAM signature, commonly used to assess photosynthetic pathway species [5]. At present CAM has been documented in diversity, does not distinguish between plants with low-levels of only 120 named species (Table 1). Most are epiphytes, a CAM and C3 plants. We provide the first census of CAM for the mere seven are terrestrial. Australian flora and suggest that the real frequency of CAM in the flora is double that currently known, with the number of Ellenberg [2] suggested that rainfall in arid Australia is too terrestrial CAM species probably 10-fold greater. Still unpredictable to support the massive water-storing suc- unresolved is the question why the large stem-succulent life — culent life-form found amongst cacti, agaves and form is absent from the native Australian flora even though euphorbs. -
Abelmoschus Moschatus Subsp
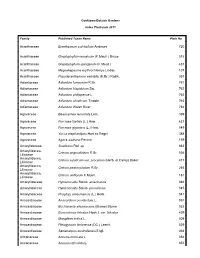
Cooktown Botanic Gardens Index Plantarum 2011 Family Published Taxon Name Plate No Acanthaceae Eranthemum pulchellum Andrews 720 Acanthaceae Graptophyllum excelsum (F.Meull.) Druce 515 Acanthaceae Graptophyllum spinigerum (F.Meull.) 437 Acanthaceae Megaskepasma erythrochlamys Lindau 107 Acanthaceae Pseuderanthemum variabile (R.Br.) Radlk. 357 Adiantaceae Adiantum formosum R.Br. 761 Adiantaceae Adiantum hispidulum Sw. 762 Adiantaceae Adiantum philippense L. 765 Adiantaceae Adiantum silvaticum Tindale 763 Adiantaceae Adiantum Walsh River 764 Agavaceae Beaucarnea recurvata Lem. 399 Agavaceae Furcraea foetida (L.) Haw. 637 Agavaceae Furcraea gigantea (L.) Haw. 049 Agavaceae Yucca elephantipes Hort.ex Regel 388 Agavaceae Agave sisalana Perrine. 159 Amarylidaceae Scadoxus Raf. sp 663 Amaryllidacea, Crinum angustifolium R.Br. 536 Liliaceae Amaryllidacea, Crinum asiaticum var. procerum (Herb. et Carey) Baker 417 Liliaceae Amaryllidacea, Crinum pedunculatum R.Br. 265 Liliaceae Amaryllidacea, Crinum uniflorum F.Muell. 161 Liliaceae Amaryllidaceae Hymenocallis Salisb. americanus 046 Amaryllidaceae Hymenocallis Salisb. peruvianna 045 Amaryllidaceae Proiphys amboinensis (L.) Herb. 041 Anacardiaceae Anacardium occidentale L. 051 Anacardiaceae Buchanania arborescens (Blume) Blume. 022 Anacardiaceae Euroschinus falcatus Hook.f. var. falcatus 429 Anacardiaceae Mangifera indica L. 009 Anacardiaceae Pleiogynium timorense (DC.) Leenh. 029 Anacardiaceae Semecarpus australiensis Engl. 368 Annonaceae Annona muricata L. 054 Annonaceae Annona reticulata L. 053 Annonaceae Annona squamosa 602 Annonaceae Cananga odorata (Lam.) Hook.f.&Thomson 406 Annonaceae Melodorum leichhardtii (F.Muell.) Diels. 360 Annonaceae Rollinia deliciosa Saff. 098 Apiaceae Centella asiatica (L.) Urb. 570 Apocynaceae Adenium obesum (Forssk.) Roem. & Schult. 489 Apocynaceae Allamanda cathartica L. 047 Apocynaceae Allamanda violacea Gardn. & Field. 048 Apocynaceae Alstonia actinophylla (A.Cunn.) K.Schum. 026 Apocynaceae Alstonia scholaris (L.) R.Br. 012 Apocynaceae Alyxia ruscifolia R.Br. -

PGR Diversity and Economic Utilization of Orchids
Int.J.Curr.Microbiol.App.Sci (2019) 8(10): 1865-1887 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 8 Number 10 (2019) Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2019.810.217 PGR Diversity and Economic Utilization of Orchids R. K. Pamarthi, R. Devadas, Raj Kumar, D. Rai, P. Kiran Babu, A. L. Meitei, L. C. De, S. Chakrabarthy, D. Barman and D. R. Singh* ICAR-NRC for Orchids, Pakyong, Sikkim, India ICAR-IARI, Kalimpong, West Bengal, India *Corresponding author ABSTRACT Orchids are one of the highly commercial crops in floriculture sector and are robustly exploited due to the high ornamental and economic value. ICAR-NRC for Orchids Pakyong, Sikkim, India, majorly focused on collection, characterization, K e yw or ds evaluation, conservation and utilization of genetic resources available in the country particularly in north-eastern region and developed a National repository of Orchids, Collection, Conservation, orchids. From 1996 to till date, several exploration programmes carried across the Utilization country and a total of 351 species under 94 genera was collected and conserved at Article Info this institute. Among the collections, 205 species were categorized as threatened species, followed by 90 species having breeding value, 87 species which are used Accepted: in traditional medicine, 77 species having fragrance and 11 species were used in 15 September 2019 traditional dietary. Successful DNA bank of 260 species was constructed for Available Online: 10 October 2019 future utilization in various research works. The collected orchid germplasm which includes native orchids was successfully utilized in breeding programme for development of novel varieties and hybrids. -

Title Floral Synomone Diversification of Sibling Bulbophyllum Species
Floral synomone diversification of sibling Bulbophyllum Title species (Orchidaceae) in attracting fruit fly pollinators Nakahira, Masataka; Ono, Hajime; Wee, Suk Ling; Tan, Keng Author(s) Hong; Nishida, Ritsuo Citation Biochemical Systematics and Ecology (2018), 81: 86-95 Issue Date 2018-12 URL http://hdl.handle.net/2433/235528 © 2018. This manuscript version is made available under the CC-BY-NC-ND 4.0 license http://creativecommons.org/licenses/by-nc-nd/4.0/.; The full- text file will be made open to the public on 01 December 2019 Right in accordance with publisher's 'Terms and Conditions for Self- Archiving'.; This is not the published version. Please cite only the published version. この論文は出版社版でありません。 引用の際には出版社版をご確認ご利用ください。 Type Journal Article Textversion author Kyoto University Floral Synomone Diversification of Bulbophyllum Sibling Species (Orchidaceae) in Attracting Fruit Fly Pollinators Masataka Nakahiraa · Hajime Onoa · Suk Ling Weeb,c · Keng Hong Tand · Ritsuo Nishidaa, * *Corresponding author Ritsuo Nishida [email protected] a Laboratory of Chemical Ecology, Graduate School of Agriculture, Kyoto University, Kyoto 606- 8502, Japan b School of Environmental and Natural Resource Sciences, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi Selangor Darul Ehsan, Malaysia c Centre for Insect Systematics, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi Selangor Darul Ehsan, Malaysia d Tan Hak Heng Co., Johor Bahru, Johor, Malaysia 1 Abstract Floral scent is one of the crucial cues to attract specific groups of insect pollinators in angiosperms. We examined the semiochemical diversity in the interactions between “fruit fly orchids” and their pollinator fruit fly species in two genera, Bactrocera and Zeugodacus (Tephritidae: Diptera). -

Review of Selected Literature and Epiphyte Classification
--------- -- ---------· 4 CHAPTER 1 REVIEW OF SELECTED LITERATURE AND EPIPHYTE CLASSIFICATION 1.1 Review of Selected, Relevant Literature (p. 5) Several important aspects of epiphyte biology and ecology that are not investigated as part of this work, are reviewed, particularly those published on more. recently. 1.2 Epiphyte Classification and Terminology (p.11) is reviewed and the system used here is outlined and defined. A glossary of terms, as used here, is given. 5 1.1 Review of Selected, Relevant Li.terature Since the main works of Schimper were published (1884, 1888, 1898), particularly Die Epiphytische Vegetation Amerikas (1888), many workers have written on many aspects of epiphyte biology and ecology. Most of these will not be reviewed here because they are not directly relevant to the present study or have been effectively reviewed by others. A few papers that are keys to the earlier literature will be mentioned but most of the review will deal with topics that have not been reviewed separately within the chapters of this project where relevant (i.e. epiphyte classification and terminology, aspects of epiphyte synecology and CAM in the epiphyt~s). Reviewed here are some special problems of epiphytes, particularly water and mineral availability, uptake and cycling, general nutritional strategies and matters related to these. Also, all Australian works of any substance on vascular epiphytes are briefly discussed. some key earlier papers include that of Pessin (1925), an autecology of an epiphytic fern, which investigated a number of factors specifically related to epiphytism; he also reviewed more than 20 papers written from the early 1880 1 s onwards. -

Studies of West Malesian Agrostophyllum Blume (Orchidaceae)
Taiwania, 57(3): 251-262, 2012 Studies of West Malesian Agrostophyllum Blume (Orchidaceae) Paul Ormerod P.O. Box 8210, Cairns 4870, Queensland, Australia. Email: [email protected] (Manuscript received 6 March 2012; accepted 20 March 2012) ABSTRACT: Studies of West Malesian material of the genus Agrostophyllum reveals that three previously described species should be treated as new synonyms of earlier named entities, namely A. arundinaceum Ridl. (= A. cyathiforme J.J.Sm.), A. mearnsii Ames (= A. globiceps Schltr.) and A. wenzelii Ames (= A. glumaceum Hook.f.). However A. formosanum Rolfe is found to be a good species, distinct from A. inocephalum (Schauer) Rchb.f. On the other hand five new taxa have been recognised and are proposed here, namely A. asahanense, A. boeeanum, A. galeandrae, A. maliauense and A. pseudolaxum. KEY WORDS: Malesia, Agrostophyllum, new species. INTRODUCTION (Holotype: AMES!). The genus Agrostophyllum Blume currently Affinis A. djaratense Schltr. sed sepalis brevioribus contains about 102 species (new taxa included) (2.75-3.80 vs. 5 mm), epichilo ovato-suborbicularis, distributed from the Seychelles to Samoa. New Guinea bipulvinatis et angustioribus (non cordatis, excavatis et is the centre of diversity with about 57 named taxa, 4.5 vs. 1.75-2.00 mm latis) differt. though my own studies indicate another 20 (including five infraspecific taxa) require description from that Roots and rhizome not seen. Stem terete basally, island. In West Malesia [Malaysia, Western Indonesia compressed above, upper half sublaxly leafy, 66.3 cm (Sumatra, Java, Kalimantan), Brunei and the long, 0.3 cm thick basally, 1.0-1.2 cm wide across Philippines] there are about 28 accepted taxa, to which upper sheaths. -

Forma Nov.: a New Peloric Orchid from Ibaraki Prefecture, Japan
The Japanese Society for Plant Systematics ISSN 1346-7565 Acta Phytotax. Geobot. 65 (3): 127–139 (2014) Cephalanthera falcata f. conformis (Orchidaceae) forma nov.: A New Peloric Orchid from Ibaraki Prefecture, Japan 1,* 1 1 HirosHi Hayakawa , CHiHiro Hayakawa , yosHinobu kusumoto , tomoko 1 1 2 3,4 nisHida , Hiroaki ikeda , tatsuya Fukuda and Jun yokoyama 1National Institute for Agro-Environmental Sciences, 3-1-3 Kannondai, Tsukuba, Ibaraki 305-8604, Japan. *[email protected] (author for correspondences); 2Faculty of Agriculture, Kochi University, Nan- koku, Kochi 783-8502, Japan; 3Faculty of Science, Yamagata University, Kojirakawa, Yamagata 990-8560, Japan; 4Institute for Regional Innovation, Yamagata University, Kaminoyama, Yamagata 999-3101, Japan We recognize a new peloric form of the orchid Cephalanthera falcata (Thunb.) Blume f. conformis Hi- ros. Hayak. et J. Yokoy., which occurs in the vicinity of the Tsukuba mountain range in Ibaraki Prefec- ture, Japan. This peloric form is sympatric with C. falcata f. falcata. The peloric flowers have a petal-like lip. The flowers are radially symmetrical and the perianth parts spread weakly compared to normal flow- ers. The stigmas are positioned at the column apex, thus neighboring stigmas and the lower parts of the pollinia are conglutinated. We observed similar vegetative traits among C. falcata f. falcata, C. falcata f. albescens S. Kobayashi, and C. falcata f. conformis. The floral morphology in C. falcata f. conformis resembles that of C. nanchuanica (S.C. Chen) X.H. Jin et X.G. Xiang (i.e., similar petal-like lip and stig- ma position at the column apex; syn. Tangtsinia nanchuanica S.C. -

Research Priorities and Future Directions in Conservation of Wild Orchids in Sri Lanka: a Review
Nature Conservation Research. Заповедная наука 2020. 5(Suppl.1): 34–45 https://dx.doi.org/10.24189/ncr.2020.029 RESEARCH PRIORITIES AND FUTURE DIRECTIONS IN CONSERVATION OF WILD ORCHIDS IN SRI LANKA: A REVIEW J. Dananjaya Kottawa-Arachchi1,*, R. Samantha Gunasekara2 1Tea Research Institute of Sri Lanka, Sri Lanka 2Lanka Nature Conservationists, Sri Lanka *e-mail: [email protected], [email protected] Received: 24.03.2020. Revised: 22.05.2020. Accepted: 29.05.2020. Together with Western Ghats, Sri Lanka is a biodiversity hotspot amongst the 35 regions known worldwide. Considering the Sri Lankan orchids, 70.6% of the orchid species, including 84% of the endemics, are categorised as threatened. The distribution of the family Orchidaceae is mostly correlated with the distribution pattern of the main bioclimatic zones which is governed by the amount and intensity of rainfall and altitude. Habitat deterioration and degradation, clearing of vegetation, intentional forest fires and spread of invasive alien species are significant threats to native species. Illegally collection and exporting of indigenous species has been another alarming issue in the past decades. Protection of native species, increased public awareness, enforcement of legislation and introduction of new propagation techniques would certainly bring a beneficial effect to the native orchid flora. Conduct awareness programs, strengthen existing laws, and reviewing the legal framework related to the native orchid flora could be vital for future conservation. Apart from the identification of new species and their distribution, future research on understanding soil chemical and physical parameters of terrestrial habitats, plant association of terrestrial orchids, phenology patterns and interactions of pollinators, associations with mycorrhiza, effect of invasive alien species and impact of climate change are highlighted.